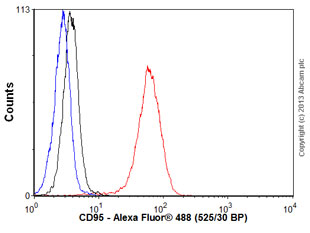
Human peripheral blood lymphocytes stained with ab109913 (red line). Human whole blood was processed using a modified protocol based on Chow et al, 2005 (PMID: 16080188). In brief, human whole blood was fixed in 4% formaldehyde (methanol-free) for 10 min at 22ºC. Red blood cells were then lyzed by the addition of Triton X-100 (final concentration - 0.1%) for 15 min at 37ºC. For experimentation, cells were treated with 50% methanol (-20ºC) for 15 min at 4ºC. Cells were then incubated with the antibody (ab109913, 0.01μg/1x106 cells) for 30 min at 4ºC. The secondary antibody used was Alexa Fluor® 488 goat anti-rabbit IgG (H&L) (ab150077) at 1/2000 dilution for 30 min at 4ºC. Isotype control antibody (black line) was rabbit IgG (polyclonal) (0.01μg/1x106 cells) used under the same conditions. Unlabelled sample (blue line) was also used as a control. Acquisition of >30,000 total events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter. Gating strategy - peri
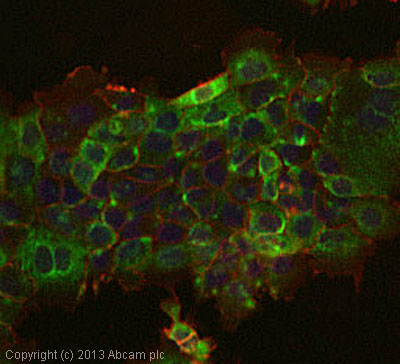
ab109913 stained A431 cells. The cells were 4% formaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody ab109913 at 5µg/ml overnight at +4°C. The secondary antibody (green) was DyLight® 488 goat anti- rabbit (ab96899) IgG (H+L) used at a 1/1000 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.

