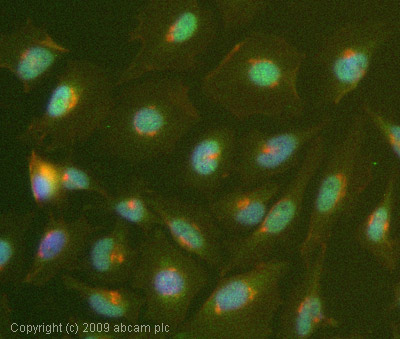
ICC/IF image of ab66498 stained HeLa cells. The cells were 4% formaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab66498, 1/1000 dilution) overnight at +4°C. The secondary antibody (green) was Alexa Fluor® 488 goat anti-rabbit IgG (H+L) used at a 1/1000 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.
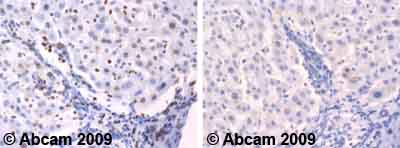
Ab66498 staining human normal liver. Staining is localised to the nucleus.Left panel: with primary antibody diluted 1:1000. Right panel: isotype control.Sections were stained using an automated system DAKO Autostainer Plus , at room temperature. Sections were rehydrated and antigen retrieved with the DAKO 3-in-1 antigen retrieval buffer citrate pH 6.0 in a DAKO PT Link. Slides were peroxidase blocked in 3% H2O2 in methanol for 10 minutes. They were then blocked with Dako Protein block for 10 minutes (containing casein 0.25% in PBS) then incubated with primary antibody for 20 minutes and detected with Dako Envision Flex amplification kit for 30 minutes. Colorimetric detection was completed with diaminobenzidine for 5 minutes. Slides were counterstained with Haematoxylin and coverslipped under DePeX. Please note that for manual staining we recommend to optimize the primary antibody concentration and incubation time (overnight incubation), and amplification may be required.

