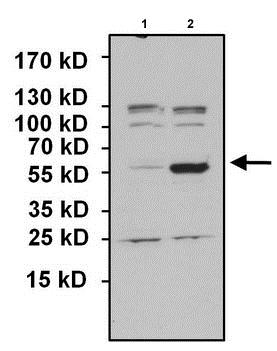
All lanes : Anti-PAX7 antibody (ab187339) at 1/1000 dilutionLane 1 : HeLa lysate transfected with empty vector controlLane 2 : HeLa lysate overexpressing PAX7Lysates/proteins at 50 µg per lane.SecondaryGoat anti-rabbit HRP at 1/20000 dilutiondeveloped using the ECL technique
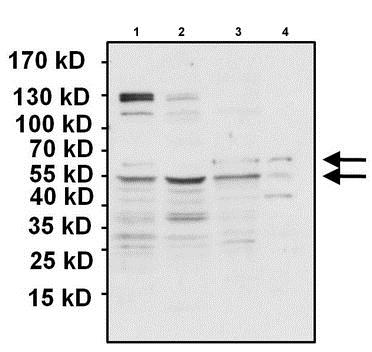
All lanes : Anti-PAX7 antibody (ab187339) at 1/1000 dilutionLane 1 : HeLa whole cell lysateLane 2 : MCF7 whole cell lysateLane 3 : HSkMSC whole cell lysateLane 4 : SJCRH30 whole cell lysateLysates/proteins at 50 µg per lane.SecondaryGoat anti-rabbit HRP at 1/20000 dilutiondeveloped using the ECL technique
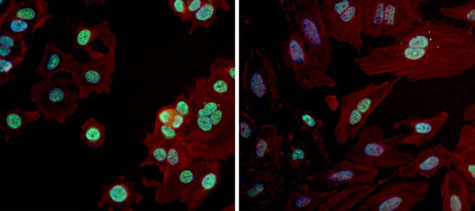
Immunofluorescent analysis of PAX7 in MCF7 (left panel) and HeLa (right panel) cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature. Cells were probed with a ab187339 at 1/100 dilution for at least 1 hour at room temperature, washed with PBS, and incubated with a DyLight 488-conjugated goat anti-rabbit IgG secondary antibody at 1/400 dilution for 30 minutes at room temperature. F-Actin (red) was stained with DyLight-554 Phalloidin and nuclei (blue) were stained with Hoechst 33342 dye.
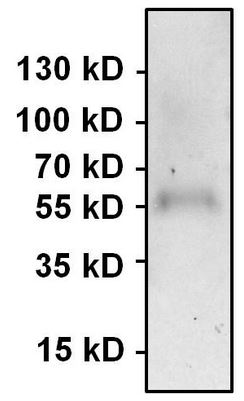
Immunoprecipitation of PAX7 was performed using HeLa whole cell lysate. Antigen-antibody complexes were formed by incubating 500µg of lysate with 3µg of ab187339 overnight on a rocking platform at 4°C. The immune complexes were captured on 50ul Protein A/G Agarose, washed extensively, and eluted. Samples was resolved on a 4-20% Tris-HCl polyacrylamide gel, transferred to a PVDF membrane, and blocked with 5% BSA/TBS-0.1%Tween-20 for 1 hour. The membrane was probed with ab187339 at 1/1000 dilution overnight rotating at 4°C.


