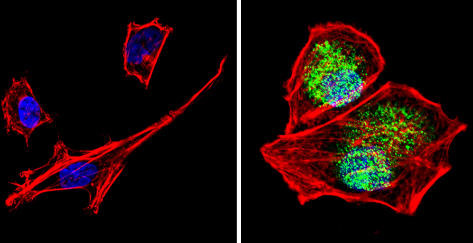
Immunocytochemistry/Immunofluorescence analysis of PER1 (green) showing staining in the cytoplasm and nucleus of SH-SY5Y cells (right) compared to a negative control (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were incubated with ab3443 in 3% BSA-PBS at a dilution of 1:100 overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
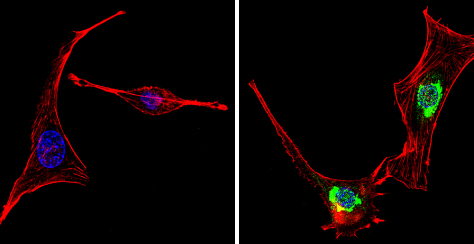
Immunocytochemsitry/Immunofluorescence analysis of PER1 (green) showing staining in the cytoplasm and nucleus of NIH-3T3 cells (right) compared to a negative control (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were incubatedwith ab3443 in 3% BSA-PBS at a dilution of 1:100 overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
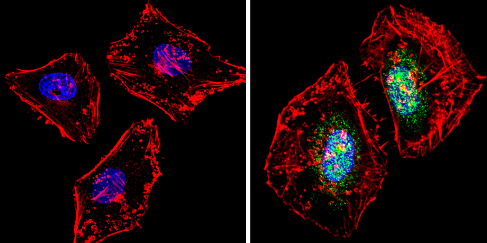
Immunocytochemsitry/Immunofluorescence analysis of PER1 (green) showing staining in the cytoplasm and nucleus of Hela cells (right) compared to a negative control (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were incubated with an3443 in 3% BSA-PBS at a dilution of 1:100 overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
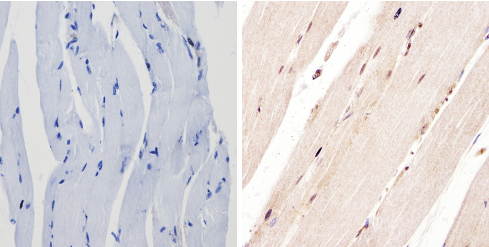
ab3443 labelling PER1 in the nucleus of Mouse skeletal muscle tissue (right) compared with a negative control (left) by Immunohistchemistry (formalin/PFA-fixed paraffin-embedded sections). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature. Tissue sections were incubated with the primary antibody (1:100 in 3% BSA-PBS) overnight at 4°C. A HRP-conjugated anti-rabbit IgG was used as the secondary antibody, followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
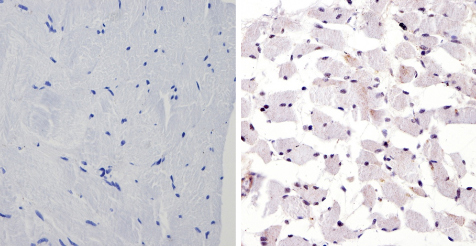
ab3443 labelling PER1 in the nucleus of Human skeletal muscle tissue (right) compared with a negative control (left) by Immunohistchemistry (formalin/PFA-fixed paraffin-embedded sections). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature. Tissue sections were incubated with the primary antibody (1:100 in 3% BSA-PBS) overnight at 4°C. A HRP-conjugated anti-rabbit IgG was used as the secondary antibody, followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
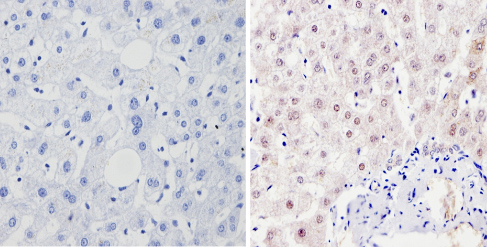
ab3443 labelling PER1 in the nucleus of Human liver tissue (right) compared with a negative control (left) by Immunohistchemistry (formalin/PFA-fixed paraffin-embedded sections). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature. Tissue sections were incubated with the primary antibody (1:100 in 3% BSA-PBS) overnight at 4°C. A HRP-conjugated anti-rabbit IgG was used as the secondary antibody, followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
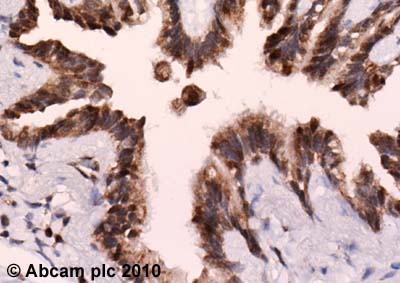
ab3443 (4µg/ml) staining PER1 in human lung using an automated system (DAKO Autostainer Plus). Using this protocol there is staining of the cytoplasm and nuclei in cells of the bronchioles.Sections were rehydrated and antigen retrieved with the Dako 3 in 1 AR buffers EDTA pH 9.0 in a DAKO PT link. Slides were peroxidase blocked in 3% H2O2 in methanol for 10 mins. They were then blocked with Dako Protein block for 10 minutes (containing casein 0.25% in PBS) then incubated with primary antibody for 20 min and detected with Dako envision flex amplification kit for 30 minutes. Colorimetric detection was completed with Diaminobenzidine for 5 minutes. Slides were counterstained with Haematoxylin and coverslipped under DePeX. Please note that, for manual staining, optimization of primary antibody concentration and incubation time is recommended. Signal amplification may be required.
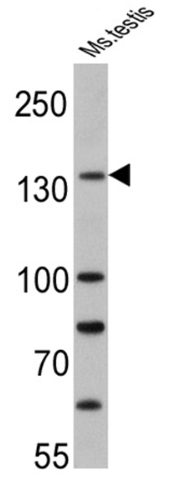
Anti-PER1 antibody (ab3443) at 1/500 dilution + Mouse testis cell lysate at 25 µg







