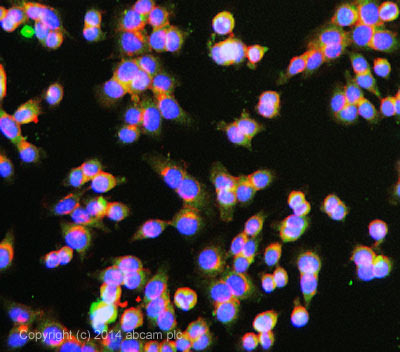
ab3765 stained HCT116 cells. The cells were 100% methanol fixed for 5 minutes at room temperature and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1hour at room temperature to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab3765 at 10µg/ml) overnight at +4°C. The secondary antibody (pseudo-colored green) was 150081 used at a 1/1000 dilution for 1hour at room temperature. Alexa Fluor® 594 WGA was used to label plasma membranes (pseudo-colored red) at a 1/200 dilution for 1hour at room temperature. DAPI was used to stain the cell nuclei (pseudo-colored blue) at a concentration of 1.43µM for 1hour at room temperature.
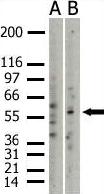
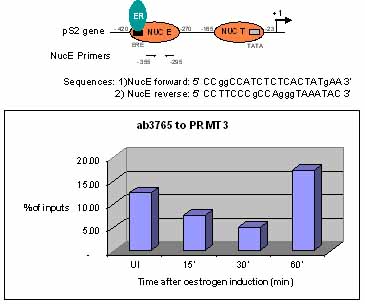
Sonicated Chromatin prepared from untreated or 17beta-estradiol (E2) treated MCF7 cells was subjected to the ChIP procedure with ab3765 to PRMT3 and the immunoprecipitated chromatin was analysed in the proximal region of the estrogen-responsive pS2 promoter (as shown above) and quantified by real-time PCR (values are % of inputs). The primers are designed to follow the nucleosome E (including the Estrogen Responsive Element ERE). 10 µl of ab3765 and 2x106 cells were used in each ChIP experiment.
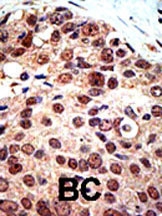
ab3765 staining PRMT3 in human breast carcinoma (BC) tissue by Immunohistochemistry (Formalin/PFA-fixed paraffin-embedded sections).



