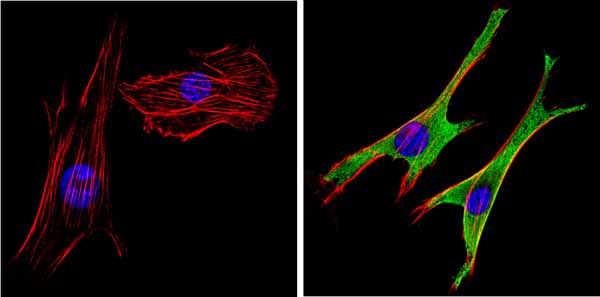
Immunocytochemistry/Immunofluorescent analysis of SAP97 (green) showing staining in the cytoplasm and membrane of NIH-3T3 cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with ab3437 in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 �C in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
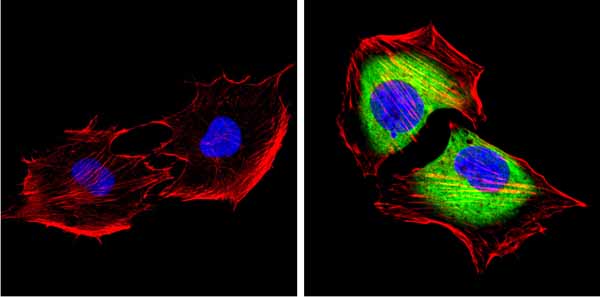
Immunocytochemistry/Immunofluorescent analysis of SAP97 (green) showing staining in the cytoplasm and membrane of C2C12 cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with ab3437 in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 �C in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
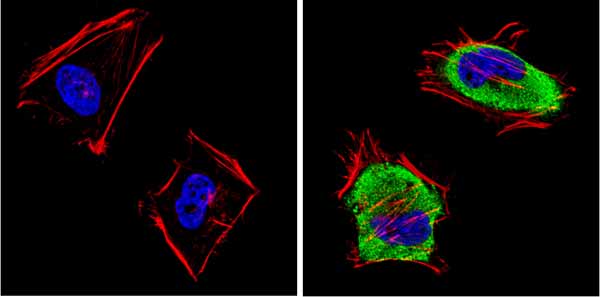
Immunocytochemistry/Immunofluorescent analysis of SAP97 (green) showing staining in the cytoplasm and membrane of HeLa cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with ab3437 in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 �C in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.

Immunofluorescent staining in the rat cortex with ab3437, rabbit polyclonal antibody to rat SAP97. Picture taken with a X40 objective. Protocol: IHC free-floating protocol using 4% PFA fixed brain tissue. Rats were intracardially perfused with 4% PFA. Tissue was post-fixed overnight in the same fixative, cryoprotected in 20% sucrose and frozen in OCT. Primary antibody ab3437 was used at 1/3000 incubated overnight at room temperature. Secondary antibody was Alexa Fluor 488 used at 1/1000, 2h incubation at room temperature. Image recoloured in Adobe photoshop.
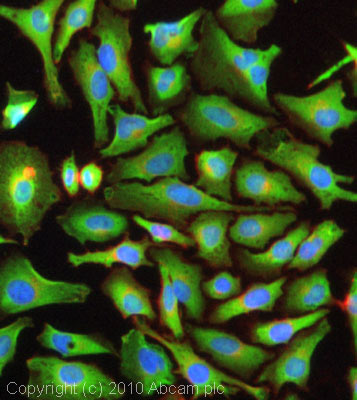
ICC/IF image of ab3437 stained HeLa cells. The cells were 4% formaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab3437, 1µg/ml) overnight at +4°C. The secondary antibody (green) was Alexa Fluor® 488 goat anti-rabbit IgG (H+L) used at a 1/1000 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.
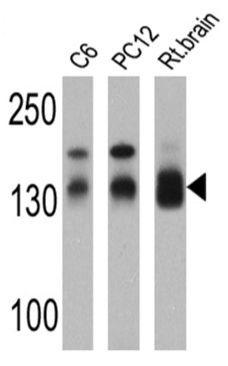
All lanes : Anti-SAP97 antibody (ab3437) at 1/5000 dilutionLane 1 : C6 cell lysateLane 2 : PC12 cell lysateLane 3 : Rat brain cell lysateLysates/proteins at 25 µg per lane.





