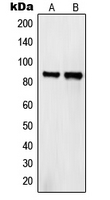
Western blot analysis of Semaphorin 4A expression in HeLa (A); COS7 (B) whole cell lysates.
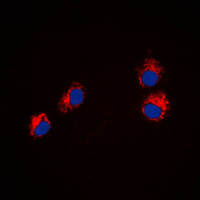
Immunofluorescent analysis of Semaphorin 4A staining in HeLa cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with the primary antibody in 3% BSA-PBS and incubated overnight at 4 C in a humidified chamber. Cells were washed with PBST and incubated with a DyLight 594-conjugated secondary antibody (red) in PBS at room temperature in the dark. DAPI was used to stain the cell nuclei (blue).

Immunoprecipitation of Semaphorin 4A from 0.5mg Jurkat whole cell extract lysate using 5ug of Anti-Semaphorin 4A Antibody and 50ul of protein G magnetic beads (+). No antibody was added to the control (-). The antibody was incubated under agitation with Protein G beads for 10min Jurkat whole cell extract lysate diluted in RIPA buffer was added to each sample and incubated for a further 10min under agitation. Proteins were eluted by addition of 40ul SDS loading buffer and incubated for 10min at 70 C; 10ul of each sample was separated on a SDS PAGE gel transferred to a nitrocellulose membrane blocked with 5% BSA and probed with Anti-Semaphorin 4A Antibody.


