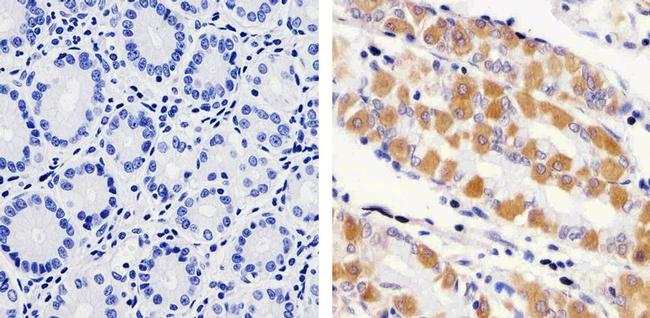
Immunohistochemistry analysis of RAB11 showing staining in the cytoplasm of paraffin-embedded human stomach tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a RAB11 polyclonal antibody (715300) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.

"Flow cytometry analysis of Rab11 was done on HeLa cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with ABfinity™ Rab11 Recombinant Rabbit Polyclonal Antibody (715300, red histogram) or with rabbit isotype control (pink histogram) at 3-5 µg/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
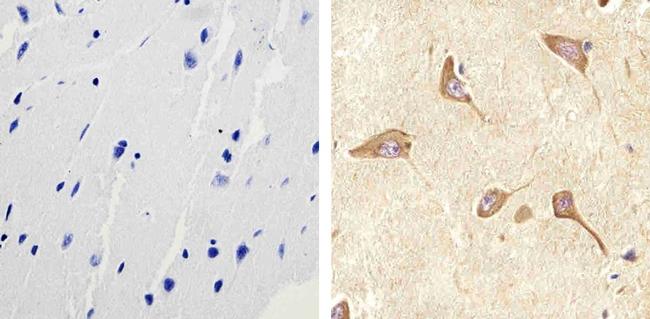
Immunohistochemistry analysis of RAB11 showing staining in the cytoplasm of paraffin-embedded human brain tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a RAB11 polyclonal antibody (715300) diluted in 3% BSA-PBS at a dilution of 1:50 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
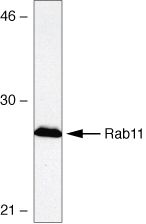
Western blot analysis of HEK293 cell lysates using Rabbit anti-Rab11 Polyclonal Antibody (Cat. No. 715300).

Western blot analysis of RAB11 was performed by loading 20 µg of Cos-7 (lane1), K562 (lane2), A431 (lane3) and SH-SY5Y (lane4) cell lysate using Novex® NuPAGE® 12 % Bis-Tris gel (NP0342BOX), XCell SureLock™ Electrophoresis System (EI0002), Novex® Sharp Pre-Stained Protein Standard (LC5800), and iBlot® Dry Blotting System (IB21001). Proteins were transferred to a nitrocellulose membrane and blocked with 5 % skim milk for 1 hour at room temperature. RAB11 was detected at ~ 27 kDa using ABfinity™ RAB11 Recombinant Rabbit Polyclonal Antibody (715300) at 1-2 µg/mL in 2.5 % skim milk at 4°C overnight on a rocking platform. Goat Anti-Rabbit IgG - HRP Secondary Antibody (G21234) at 1:5000 dilution was used and chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (WP20005).

Immunofluorescence analysis of Rab11 was done on 70% confluent log phase U2OS cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with Rab11 Rabbit Polyclonal Antibody (715300) at 2 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant DAPI (S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (A12381). Panel d is a merged image showing cytoplasmic localization. Panel e shows no primary antibody control. The images were captured at 20X magnification.
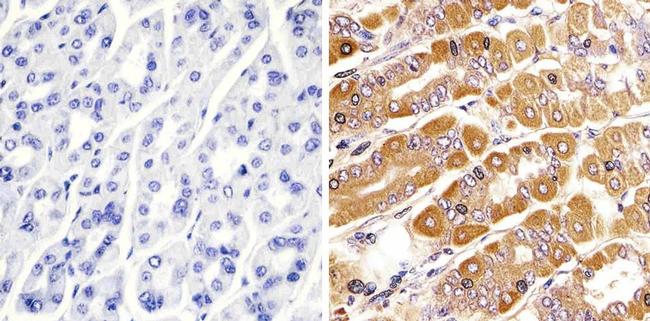
Immunohistochemistry analysis of RAB11 showing staining in the cytoplasm of paraffin-embedded mouse stomach tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a RAB11 polyclonal antibody (715300) diluted in 3% BSA-PBS at a dilution of 1:50 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.






