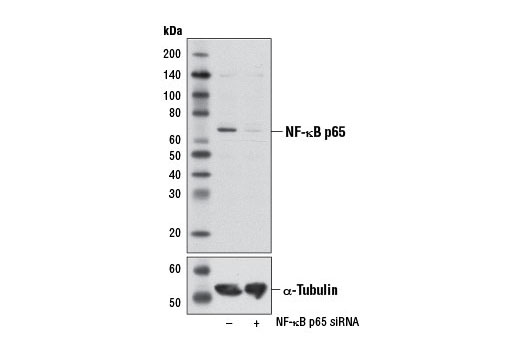
Western blot analysis of extracts from HeLa cells, transfected with 100 nM SignalSilence ® Control siRNA (Unconjugated) #6568 (-) or SignalSilence ® NF-κB p65 siRNA I #6261 (+), using NF-κB p65 (L8F6) Mouse mAb (upper) or α-Tubulin (11Η10) Rabbit mAb #2125 (lower). The NF-κB p65 (L8F6) Mouse mAb confirms silencing of NF-κB p65 expression, while the α-Tubulin (11Η10) Rabbit mAb is used as a loading control.

Western blot analysis of extracts from various cell lines using NF-κB p65 (L8F6) Mouse mAb.

Immunohistochemical analysis of human chronic cholecystitis tissue using NF-κB p65 (L8F6) Mouse mAb.

Immunohistochemical analysis of paraffin-embedded OVCAR8 cell pellets treated with Human Tumor Necrosis Factor-α (hTNF-α) #8902 (left) or treated with SignalSilence ® NF-κB p65 siRNA I #6261 (right), using NF-κB p65 (L8F6) Mouse mAb.

Immunohistochemical analysis of paraffin-embedded HeLa cell pellets, untreated (left) or treated with Human Tumor Necrosis Factor-α (hTNF-α) #8902 (right), using NF-κB p65 (L8F6) Mouse mAb.

Confocal immunofluorescent analysis of HeLa cells, untreated (left) or treated with Human Tumor Necrosis Factor-α (hTNF-α) #8902 (20 ng/mL, 20 min; right), using NF-κB p65 (L8F6) Mouse mAb (green). Actin filaments were labeled with DY-554 phalloidin (red). Blue pseudocolor = DRAQ5 ® #4084 (fluorescent DNA dye).

Flow cytometric analysis of HeLa cells using NF-κB p65 (L8F6) Mouse mAb (blue) compared to a nonspecific negative control antibody (red).

Chromatin immunoprecipitations were performed with cross-linked chromatin from 4 x 10 6 HeLa cells treated with Human Tumor Necrosis Factor-α (hTNF-α) #8902 (30 ng/ml, 1 hr) and either 10 μl of NF-κB p65 (L8F6) Mouse mAb or 2 μl of Normal Rabbit IgG #2729 using SimpleChIP ® Enzymatic Chromatin IP Kit (Magnetic Beads) #9003. The enriched DNA was quantified by real-time PCR using SimpleChIP ® Human IκBα Promoter Primers #5552, human IL-8 promoter primers, and SimpleChIP ® Human α Satellite Repeat Primers #4486. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one.







