Anti-Acetylated alpha Tubulin antibody [6-11B-1]
| Name | Anti-Acetylated alpha Tubulin antibody [6-11B-1] |
|---|---|
| Supplier | Abcam |
| Catalog | ab24610 |
| Prices | $403.00 |
| Sizes | 100 µl |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG2b |
| Clone | 6-11B-1 |
| Applications | FC WB ICC/IF IHC-P ICC/IF ICC/IF IHC-F |
| Species Reactivities | Mouse, Rat, Sheep, Human, Monkey, Sea Urchin, Bovine |
| Antigen | Tissue/ cell preparation - acetylated alpha-tubulin from the axoneme of sea urchin sperm flagella |
| Description | Mouse Monoclonal |
| Gene | TUBA1B |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
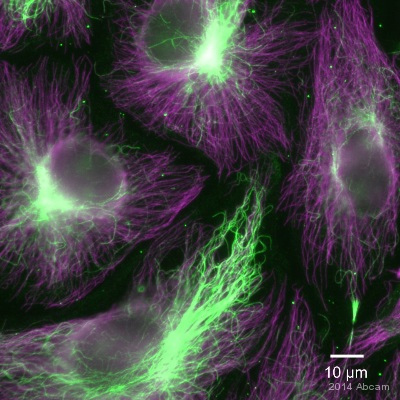
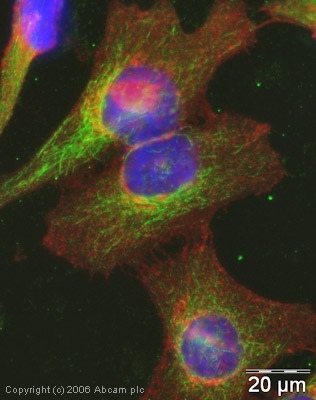
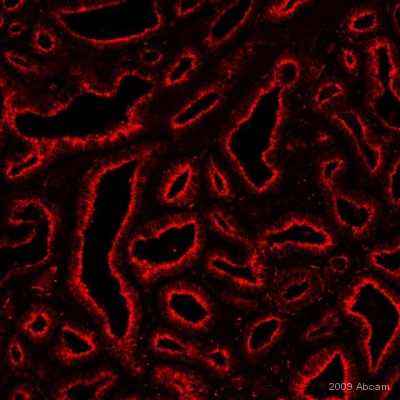
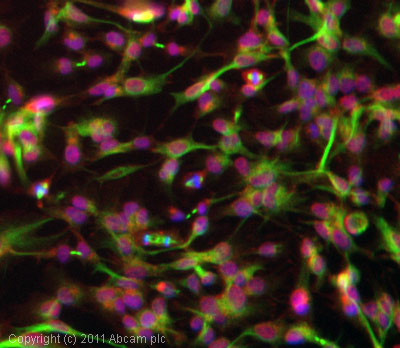
Product images
Product References
Effects of vitamin D on airway epithelial cell morphology and rhinovirus - Effects of vitamin D on airway epithelial cell morphology and rhinovirus
Brockman-Schneider RA, Pickles RJ, Gern JE. PLoS One. 2014 Jan 24;9(1):e86755.
Mechanism of cystogenesis in nephrotic kidneys: a histopathological study. - Mechanism of cystogenesis in nephrotic kidneys: a histopathological study.
Saraga M, Vukojevic K, Krzelj V, Puretic Z, Bocina I, Durdov MG, Weber S, Dworniczak B, Ljubanovic DG, Saraga-Babic M. BMC Nephrol. 2014 Jan 8;15:3.
A conserved flagella-associated protein in Chlamydomonas, FAP234, is essential - A conserved flagella-associated protein in Chlamydomonas, FAP234, is essential
Kubo T, Yanagisawa HA, Liu Z, Shibuya R, Hirono M, Kamiya R. Mol Biol Cell. 2014 Jan;25(1):107-17.
A phase I, pharmacokinetic, and pharmacodynamic study of panobinostat, an HDAC - A phase I, pharmacokinetic, and pharmacodynamic study of panobinostat, an HDAC
Gray JE, Haura E, Chiappori A, Tanvetyanon T, Williams CC, Pinder-Schenck M, Kish JA, Kreahling J, Lush R, Neuger A, Tetteh L, Akar A, Zhao X, Schell MJ, Bepler G, Altiok S. Clin Cancer Res. 2014 Mar 15;20(6):1644-55.
Role of tumor necrosis factor alpha-induced protein 1 in paclitaxel resistance. - Role of tumor necrosis factor alpha-induced protein 1 in paclitaxel resistance.
Zhu Y, Yao Z, Wu Z, Mei Y, Wu M. Oncogene. 2014 Jun 19;33(25):3246-55.
The membrane proteome of sensory cilia to the depth of olfactory receptors. - The membrane proteome of sensory cilia to the depth of olfactory receptors.
Kuhlmann K, Tschapek A, Wiese H, Eisenacher M, Meyer HE, Hatt HH, Oeljeklaus S, Warscheid B. Mol Cell Proteomics. 2014 Jul;13(7):1828-43.
Expression of Ki-67, Oct-4, gamma-tubulin and alpha-tubulin in human tooth - Expression of Ki-67, Oct-4, gamma-tubulin and alpha-tubulin in human tooth
Kero D, Novakovic J, Vukojevic K, Petricevic J, Kalibovic Govorko D, Biocina-Lukenda D, Saraga-Babic M. Arch Oral Biol. 2014 Nov;59(11):1119-29.
Cby1 promotes Ahi1 recruitment to a ring-shaped domain at the centriole-cilium - Cby1 promotes Ahi1 recruitment to a ring-shaped domain at the centriole-cilium
Lee YL, Sante J, Comerci CJ, Cyge B, Menezes LF, Li FQ, Germino GG, Moerner WE, Takemaru K, Stearns T. Mol Biol Cell. 2014 Oct 1;25(19):2919-33.
The Rilp-like proteins Rilpl1 and Rilpl2 regulate ciliary membrane content. - The Rilp-like proteins Rilpl1 and Rilpl2 regulate ciliary membrane content.
Schaub JR, Stearns T. Mol Biol Cell. 2013 Feb;24(4):453-64.
Chronic IL9 and IL-13 exposure leads to an altered differentiation of ciliated - Chronic IL9 and IL-13 exposure leads to an altered differentiation of ciliated
Parker JC, Thavagnanam S, Skibinski G, Lyons J, Bell J, Heaney LG, Shields MD. PLoS One. 2013 May 9;8(5):e61023.
![All lanes : Anti-Acetylated alpha Tubulin antibody [6-11B-1] (ab24610) at 5 µg/mlLane 1 : HeLa (Human epithelial carcinoma cell line) Whole Cell Lysate Lane 2 : NIH 3T3 (Mouse embryonic fibroblast cell line) Whole Cell Lysate Lane 3 : PC12 (Rat adrenal pheochromocytoma cell line) Whole Cell Lysate Lane 4 : Brain (Mouse) Tissue Lysate Lysates/proteins at 20 µg per lane.SecondaryGoat Anti-Mouse IgG H&L (HRP) preadsorbed (ab97040) at 1/5000 dilutionPerformed under reducing conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/1704_Acetylated-alpha-Tubulin-Primary-antibodies-ab24610-20.jpg)
![Overlay histogram showing HeLa cells stained with ab24610 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab24610, 1µg/1x106 cells) for 30 min at 22ºC. (This data was generated from a purified version of the antibody. Some lots are produced as ascites fluid. We suggest 1µl/1x106 cells for ascites preparations). The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG2b [PLPV219] (ab91366, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed. This antibody gave a positive signal in HeLa cells fixed with 80% methanol (5 min)/permeabilized with 0.1% PBS-Tween for 20 min used under the same conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/1709_Acetylated-alpha-Tubulin-Primary-antibodies-ab24610-23.jpg)