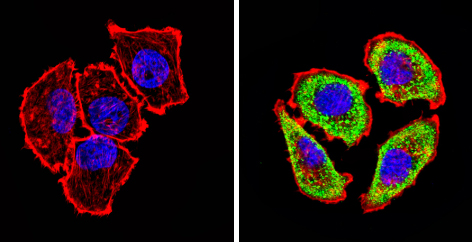
Immunocytochemistry/Immunofluorescence analysis of Adenosine Receptor A2a (green) showing staining in the cytoplasm of U251 cells (right) compared to a negative control (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were incubated with ab3461 in 3% BSA-PBS at a dilution of 1:20 overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
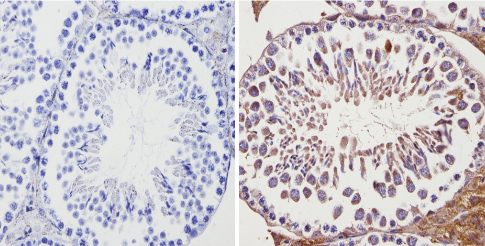
ab3461 labelling Adenosine Receptor A2a in the cytoplasm and membrane of Mouse testis tissue (right) compared with a negative control (left) by Immunohistochemisty (formalin/PFA-fixed paraffin-embedded sections). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature. Tissue sections were incubated with the primary antibody (1:20 in 3% BSA-PBS) overnight at 4°C. A HRP-conjugated anti-rabbit IgG was as the secondary antibody, followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
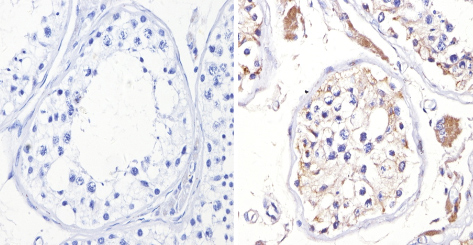
ab3461 labelling Adenosine Receptor A2a in the cytoplasm and membrane of Human testis tissue (right) compared with a negative control (left) by Immunohistochemisty (formalin/PFA-fixed paraffin-embedded sections). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature. Tissue sections were incubated with the primary antibody (1:200 in 3% BSA-PBS) overnight at 4°C. A HRP-conjugated anti-rabbit IgG was as the secondary antibody, followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
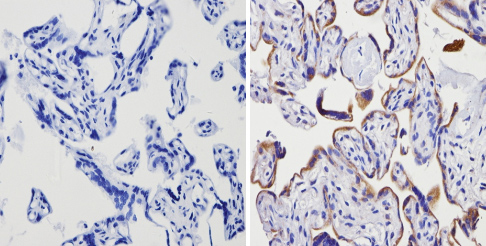
ab3461 labelling Adenosine Receptor A2a in the cytoplasm and membrane of Human placenta tissue (right) compared with a negative control (left) by Immunohistochemisty (formalin/PFA-fixed paraffin-embedded sections). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature. Tissue sections were incubated with the primary antibody (1:100 in 3% BSA-PBS) overnight at 4°C. A HRP-conjugated anti-rabbit IgG was as the secondary antibody, followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.

Anti-Adenosine Receptor A2a antibody (ab3461) at 1/1000 dilution + Human heart tissue lysate at 20 µgSecondaryDonkey HRP-conjugate anti-rabbit at 1/5000 dilutiondeveloped using the ECL techniquePerformed under reducing conditions.Exposure time : 3 minutesThis image is courtesy of an anonymous AbreviewSee Abreview

All lanes : Anti-Adenosine Receptor A2a antibody (ab3461) at 1/500 dilutionLane 1 : Human placenta cell lysateLane 2 : HepG2 cell lysateLane 3 : HeLa cell lysateLane 4 : Mouse liver cell lysateLysates/proteins at 25 µg per lane.
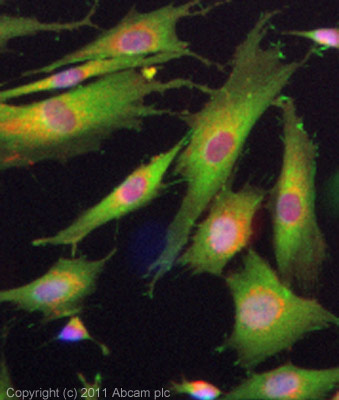
ICC/IF image of ab3461 stained SKNSH cells. The cells were 100% methanol fixed (5 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab3461, 10µg/ml) overnight at +4°C. The secondary antibody (green) was ab96899 Dylight 488 goat anti-rabbit IgG (H+L) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.






