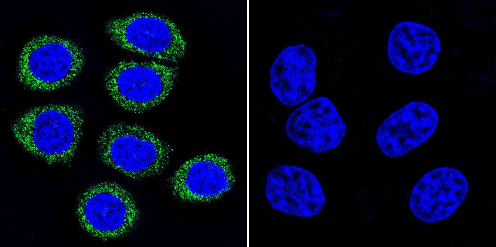
Immunocytochemistry/Immunofluorescence analysis of ADP Ribosylation Factor shows staining in HeLa cells. ADP Ribosylation Factor (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were incubated without (control) or with ab2806 (1:100) overnight at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody. Images were taken at 60X magnification.
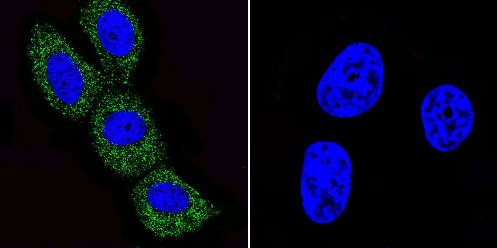
Immunocytochemistry/Immunofluorescence analysis of ADP Ribosylation Factor shows staining in MCF-7 cells. ADP Ribosylation Factor (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were incubated without (control) or with ab2806 (1:100) overnight at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody. Images were taken at 60X magnification.
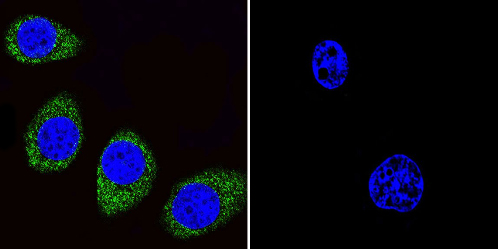
Immunocytochemistry/Immunofluorescence analysis of ADP Ribosylation Factor shows staining in U251 cells. ADP Ribosylation Factor (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were incubated without (control) or with ab2806 (1:100) overnight at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody. Images were taken at 60X magnification.
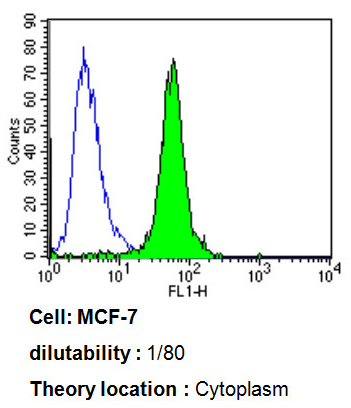
Flow cytometry analysis of ADP Ribosylation Factor showing positive staining in the cytoplasm of MCF-7 cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/ml, fixed with 2% paraformaldehyde, washed with PBS, and incubated with ab2806 (1:80) for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
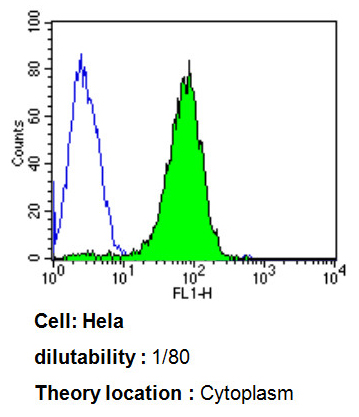
Flow cytometry analysis of ADP Ribosylation Factor showing positive staining in the cytoplasm of Hela cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/ml, fixed with 2% paraformaldehyde, washed with PBS, and incubated with ab2806 (1:80) for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
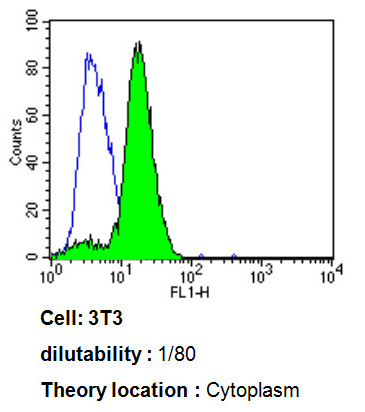
Flow cytometry analysis of ADP Ribosylation Factor showing positive staining in the cytoplasm of 3T3 cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/ml, fixed with 2% paraformaldehyde, washed with PBS, and incubated with ab2806 (1:80) for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
![Anti-ADP Ribosylation Factor antibody [1D9] (ab2806) + Canine heart extract](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/3307_ab2806-219147-ab2806wb.jpg)
Anti-ADP Ribosylation Factor antibody [1D9] (ab2806) + Canine heart extract
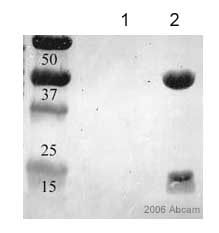
Ab2806 immunoprecipitating ADP Ribosylation Factor from HeLa cells. 10ul of antibody were added to 500ul of HeLa cell lysate (at 1.5mg/ml) and incubated overnight before precipitation with Protein G. Negative control lysate received the same treatment except no antibody was added. ab2806 was used at 1/500 in the western blotting stage and the secondary antibody was an HRP conjugated anti-mouse IgG. The membrane was finally stained with amido black.Lane 1: negative controlLane 2: ab2806This image is courtesy of an Abreview submitted by Denis Krndija submitted on 5 January 2006.See Abreview
![All lanes : Anti-ADP Ribosylation Factor antibody [1D9] (ab2806) at 1/500 dilutionLane 1 : Lysates prepared from human Huh-7 cellsLane 2 : Lysates prepared from human Huh-7 cellsLysates/proteins at 15000 cells per lane.SecondaryHRP-conjugated sheep polyclonal to mouse IgG at 1/10000 dilutionPerformed under reducing conditions.Observed band size : 21 kDa (why is the actual band size different from the predicted?)Exposure time : 4 minutesThis image is a courtesy of Anonymous AbreviewSee Abreview](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/3309_ADP-Ribosylation-Factor-Primary-antibodies-ab2806-4.jpg)
All lanes : Anti-ADP Ribosylation Factor antibody [1D9] (ab2806) at 1/500 dilutionLane 1 : Lysates prepared from human Huh-7 cellsLane 2 : Lysates prepared from human Huh-7 cellsLysates/proteins at 15000 cells per lane.SecondaryHRP-conjugated sheep polyclonal to mouse IgG at 1/10000 dilutionPerformed under reducing conditions.Observed band size : 21 kDa (why is the actual band size different from the predicted?)Exposure time : 4 minutesThis image is a courtesy of Anonymous AbreviewSee Abreview
![Overlay histogram showing HeLA cells stained with ab2806 (red line). The cells were fixed with 100% methanol (5 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab2806, 1/20 dilution) for 30 min at 22°C. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22°C. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/3310_ADP-Ribosylation-Factor-Primary-antibodies-ab2806-5.jpg)
Overlay histogram showing HeLA cells stained with ab2806 (red line). The cells were fixed with 100% methanol (5 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab2806, 1/20 dilution) for 30 min at 22°C. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22°C. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed.
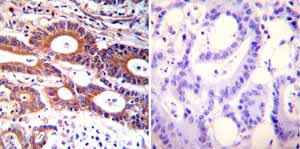
Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human colon carcinoma tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a mouse monoclonal antibody recognizing ADP-Ribosylation Factor ab2806 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
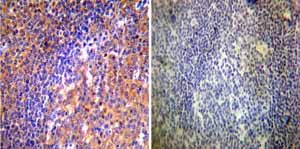
Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human tonsil tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:50 with a mouse monoclonal antibody recognizing ADP-Ribosylation Factor ab2806 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
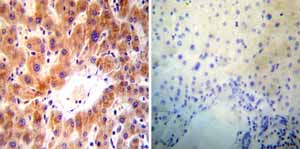
Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human liver tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:50 with a mouse monoclonal antibody recognizing ADP-Ribosylation Factor ab2806 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.






![Anti-ADP Ribosylation Factor antibody [1D9] (ab2806) + Canine heart extract](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/3307_ab2806-219147-ab2806wb.jpg)

![All lanes : Anti-ADP Ribosylation Factor antibody [1D9] (ab2806) at 1/500 dilutionLane 1 : Lysates prepared from human Huh-7 cellsLane 2 : Lysates prepared from human Huh-7 cellsLysates/proteins at 15000 cells per lane.SecondaryHRP-conjugated sheep polyclonal to mouse IgG at 1/10000 dilutionPerformed under reducing conditions.Observed band size : 21 kDa (why is the actual band size different from the predicted?)Exposure time : 4 minutesThis image is a courtesy of Anonymous AbreviewSee Abreview](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/3309_ADP-Ribosylation-Factor-Primary-antibodies-ab2806-4.jpg)
![Overlay histogram showing HeLA cells stained with ab2806 (red line). The cells were fixed with 100% methanol (5 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab2806, 1/20 dilution) for 30 min at 22°C. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22°C. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/3310_ADP-Ribosylation-Factor-Primary-antibodies-ab2806-5.jpg)


