![Human peripheral blood lymphocytes stained with ab59475 (red line). Human whole blood was processed using a modified protocol based on Chow et al, 2005 (PMID: 16080188). In brief, human whole blood was fixed in 4% formaldehyde (methanol-free) for 10 min at 22°C. Red blood cells were then lyzed by the addition of Triton X-100 (final concentration - 0.1%) for 15 min at 37°C. For experimentation, cells were treated with 50% methanol (-20°C) for 15 min at 4°C. Cells were then incubated with the antibody (ab59475, 0.01μg/1x106 cells) for 30 min at 4°C. The secondary antibody used was Alexa Fluor® 488 goat anti-mouse IgG (H&L) (ab150113) at 1/2000 dilution for 30 min at 4°C. Isotype control antibody (black line) was mouse IgG2a [ICIGG2A] (ab91361, 0.1μg/1x106 cells) used under the same conditions. Unlabelled sample (blue line) was also used as a control. Acquisition of >30,000 total events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter. Gating strategy - perip](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/25358_ab59475-3-ab59475FC.jpg)
Human peripheral blood lymphocytes stained with ab59475 (red line). Human whole blood was processed using a modified protocol based on Chow et al, 2005 (PMID: 16080188). In brief, human whole blood was fixed in 4% formaldehyde (methanol-free) for 10 min at 22°C. Red blood cells were then lyzed by the addition of Triton X-100 (final concentration - 0.1%) for 15 min at 37°C. For experimentation, cells were treated with 50% methanol (-20°C) for 15 min at 4°C. Cells were then incubated with the antibody (ab59475, 0.01μg/1x106 cells) for 30 min at 4°C. The secondary antibody used was Alexa Fluor® 488 goat anti-mouse IgG (H&L) (ab150113) at 1/2000 dilution for 30 min at 4°C. Isotype control antibody (black line) was mouse IgG2a [ICIGG2A] (ab91361, 0.1μg/1x106 cells) used under the same conditions. Unlabelled sample (blue line) was also used as a control. Acquisition of >30,000 total events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter. Gating strategy - perip
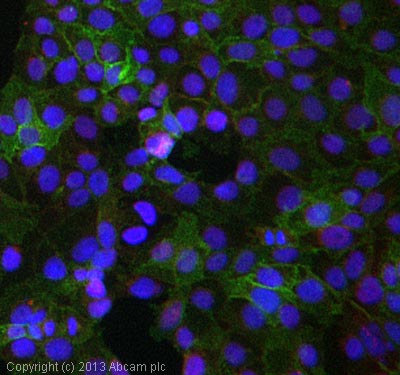
ICC/IF image of ab59475 stained Jeg3 cells. The cells were 4% formaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab59475, 10µg/ml) overnight at +4°C. The secondary antibody (green) was ab96879, DyLight® 488 goat anti-mouse IgG (H+L) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM
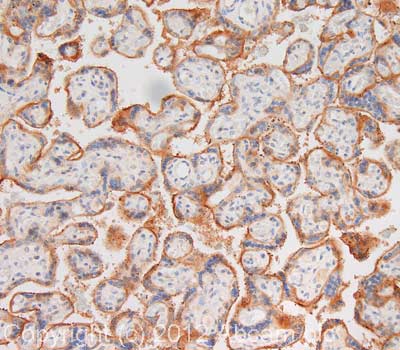
IHC image of CD59 staining in human placenta formalin fixed paraffin embedded tissue section, performed on a Leica BondTM system using the standard protocol F. The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH6, epitope retrieval solution 1) for 20 mins. The section was then incubated with ab59475, 5µg/ml, for 15 mins at room temperature and detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The section was then counterstained with haematoxylin and mounted with DPX. For other IHC staining systems (automated and non-automated) customers should optimize variable parameters such as antigen retrieval conditions, primary antibody concentration and antibody incubation times.
![Human peripheral blood lymphocytes stained with ab59475 (red line). Human whole blood was processed using a modified protocol based on Chow et al, 2005 (PMID: 16080188). In brief, human whole blood was fixed in 4% formaldehyde (methanol-free) for 10 min at 22°C. Red blood cells were then lyzed by the addition of Triton X-100 (final concentration - 0.1%) for 15 min at 37°C. For experimentation, cells were treated with 50% methanol (-20°C) for 15 min at 4°C. Cells were then incubated with the antibody (ab59475, 0.01μg/1x106 cells) for 30 min at 4°C. The secondary antibody used was Alexa Fluor® 488 goat anti-mouse IgG (H&L) (ab150113) at 1/2000 dilution for 30 min at 4°C. Isotype control antibody (black line) was mouse IgG2a [ICIGG2A] (ab91361, 0.1μg/1x106 cells) used under the same conditions. Unlabelled sample (blue line) was also used as a control. Acquisition of >30,000 total events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter. Gating strategy - perip](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/25358_ab59475-3-ab59475FC.jpg)

