Anti-Claudin 3 antibody
| Name | Anti-Claudin 3 antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab15102 |
| Prices | $400.00 |
| Sizes | 500 µl |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | ICC/IF ICC/IF WB IHC-P |
| Species Reactivities | Mouse, Rat, Chicken, Human, Pig |
| Antigen | Synthetic peptide (Mouse) (C terminal) |
| Description | Rabbit Polyclonal |
| Gene | CLDN3 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
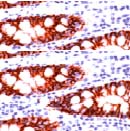
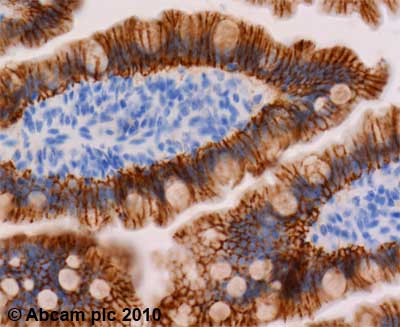
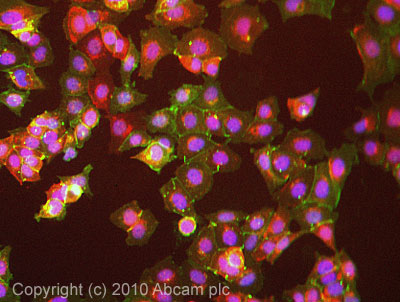
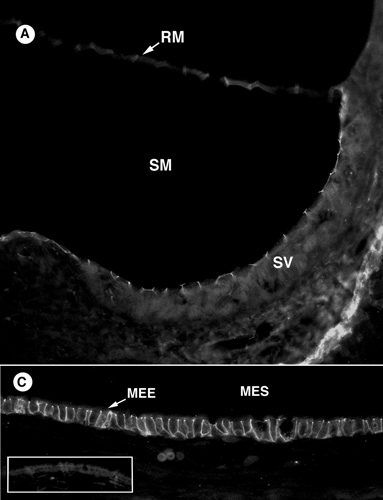
Product images
Product References
Mouse middle ear ion homeostasis channels and intercellular junctions. - Mouse middle ear ion homeostasis channels and intercellular junctions.
Morris LM, DeGagne JM, Kempton JB, Hausman F, Trune DR. PLoS One. 2012;7(6):e39004.
Blood-testis barrier dynamics are regulated by testosterone and cytokines via - Blood-testis barrier dynamics are regulated by testosterone and cytokines via
Yan HH, Mruk DD, Lee WM, Cheng CY. FASEB J. 2008 Jun;22(6):1945-59.
Chronic metabolic acidosis upregulated claudin mRNA expression in the duodenal - Chronic metabolic acidosis upregulated claudin mRNA expression in the duodenal
Charoenphandhu N, Wongdee K, Tudpor K, Pandaranandaka J, Krishnamra N. Life Sci. 2007 Apr 17;80(19):1729-37. Epub 2007 Mar 2.