
Anti-DAGLA antibody (ab81984) at 0.3 µg/ml + human liver lysate (in RIPA buffer) at 35 µgdeveloped using the ECL technique
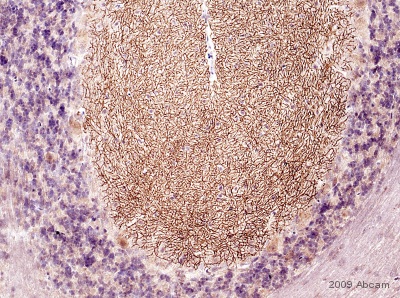
Immunohistochemistical detection of DAGLA using ab81984 on formaldehyde fixed rat cerebellum tissue sections (cerebellum). Antigen retrieval step: heat mediated in citric acid pH6. 1%BSA as blocking agent for 10 mins@ rt°C. Primary antibody dilution 1/1000 for 2 hours. Secondary antibody: anti-sheep IgG conjugated to biotin (1/200). The pattern of immunolabelling is very similar to that obtained in mouse. However, the cell bodies of some Purkinje cells are also positive, this appears to be punctate and evenly distributed.See Abreview
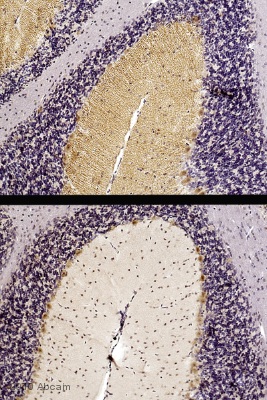
Immunohistochemistical detection of DAGLA with ab81984 on formaldehyde fixed mouse cerebellum sections. Antigen retrieval step: heat mediated in citric acid pH6. 1% BSA used as blocking agent for 10 mins @ rt°C. Primary antibody ab81984 incubated at 1/500 for 2 hours. Secondary antibody: anti sheep IgG conjugated to biotin (1/200). The upper image shows DAGL immunostaining with ab81984 on wildtype mouse cerebellum, the lower image shows an absence of DAGL alpha immunostaining on knockout mouse mouse cerebellum. These results are consistent with published data with other anti-DAGL alpha antibodies (http://jcb.rupress.org/cgi/content/full/163/3/463)See Abreview
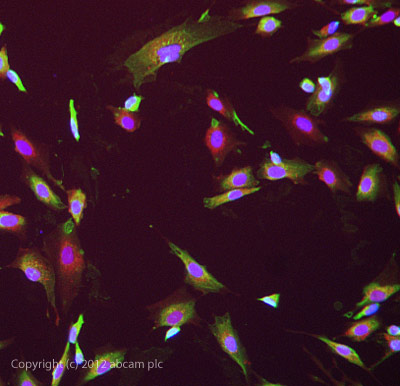
ab81984 stained SKNSH cells. The cells were 4% formaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal donkey serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody ab81984 at 10µg/ml overnight at +4°C. The secondary antibody (green) was DyLight® 488 donkey anti- goat IgG (H+L) used at a 1/1000 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.



