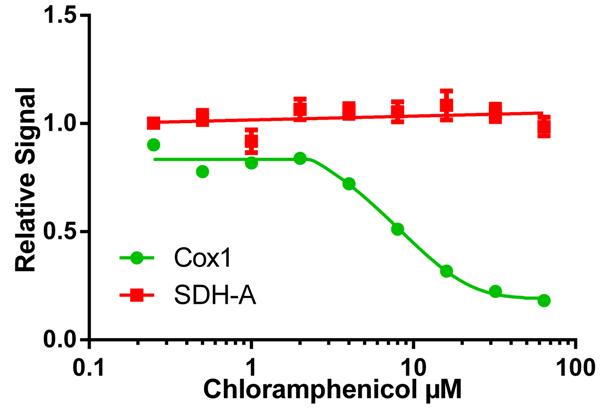
The IC50 of a drug’s effect on mitochondrial protein translation can be determined quickly using the MitoBiogenesis™ ICE Kit. In this example, cells were seeded at 6,000 cells/well, allowed to grow for 6 days in a drug dilution series and then relative amounts of COX-I (HRP signal), and SDH-A (AP-signal) were measured in each well. Chloramphenicol inhibits mtDNA-encoded (HRP signal) COX-I protein synthesis relative to nuclear DNA-encoded (AP signal) SDH-A protein synthesis by 50% at 8.1 µM, %CV = 4.33% for HRP (COX-1) signal and 3.13% for AP (SDH-A) signal.
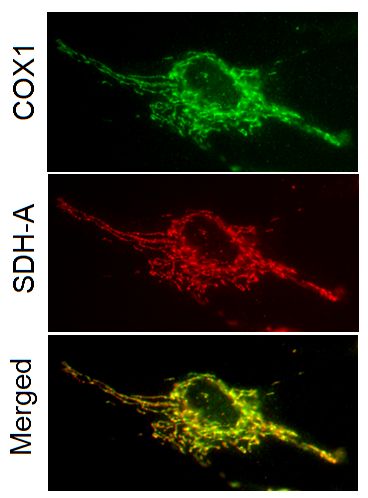
Two-color immunocytochemical labeling of cultured cells with the two primary monoclonal antibodies specific for COX-I and SDH-A. The two antibodies exhibit striking and specific co-localization in the mitochondria, consistent with the known mitochondrial expression of both proteins.
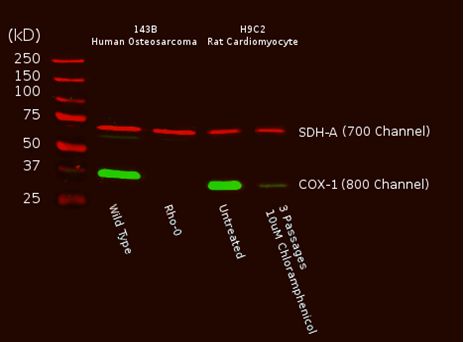
A Western blot of total cell protein (10 µg) from Human or rat cultured cells was probed with the primary and secondary antibodies and scanned with a LI-COR® Odyssey® imager. The two mitochondrial proteins targeted by the two primary mAbs were labeled and visualized specifically despite the presence of thousands of other proteins. Furthermore, reduction of mtDNA levels in human Rho0 (mtDNA-depleted) cells, or inhibition of mitochondrial protein translation by chloramphenicol in rat cells result in specific reduction of COX-I protein while nuclear DNA-encoded SDH-A is unaffected
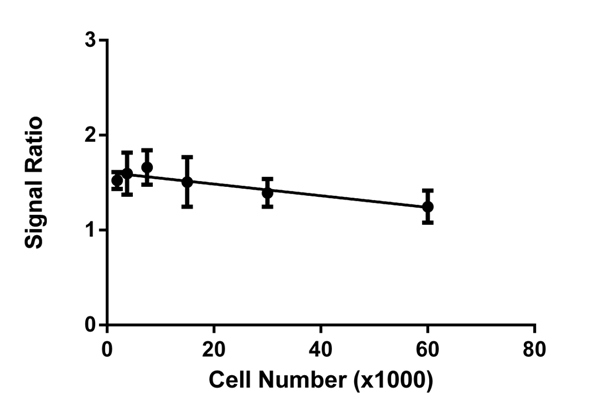
At all cell concentrations, a consistent ratio of mtDNA-encoded protein expression COX-I (HRP signal) to nuclear DNA-encoded mitochondrial protein expression SDH-A (AP signal) is observed in untreated cells. Therefore, normalizing COX-I levels to SDH-A levels simplifies data analysis and eliminates the need to perform all tests at the same cell concentration.



