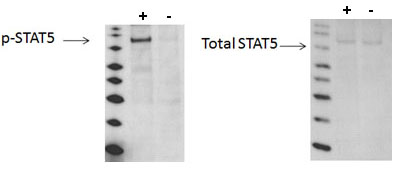
Using Western blot, a significant stimulation of STAT5 phosphorylation at Tyr694/699 is detected in A431 cells treated with EGF for 10 minutes (+) compared with cells treated with AG1478 (-).
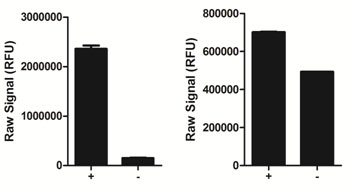
Using the phospho-STAT5 assay kit, a significant stimulation of STAT5 phosphorylation at Tyr694/699 is detected in A431 cells treated with EGF for 10 minutes (+) compared with cells treated with AG1478 (-).
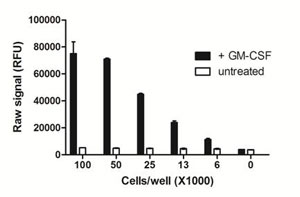
Using the PhosphoTracer phospho-STAT5 all-in-one-well assay protocol, the amount of THP-1 cells required to detect STAT5 in GM-CSF-treated cells was examined. THP-1 cells were resuspended at 5x106 cells/mL in HBSS/5% FBS, and incubated at 37°C for 1 h. Cells (20 µL) were then seeded at various densities in a rinsed PhosphoTracer assay plate, and stimulated with 20 µL of 20 ng/mL GM-CSF for 10 mins. Cells were lysed using freshly prepared Lysis Mix Concentrate, with shaking for 10 min, and analyzed for phospho-STAT5 using the PhosphoTracer protocol.
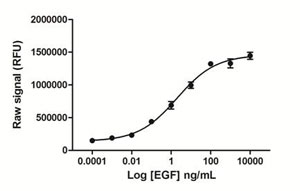
Day 1: A431 cells were seeded at 25K/well in a 96 well tissue culture plate in medium containing 10% FBS. Day 2: The cells were stimulated with various concentrations of EGF for 15 minutes. The media was removed, and the cells were lysed with 125 µL/well freshly prepared Lysis Mix, with shaking for 10 min. Lysates (50 µL) were transferred to a PhosphoTracer assay plate and analyzed for either phospho STAT5 using the standard PhosphoTracer protocol. Briefly, Antibody Mix specific for phospho-STAT5 (50 µL/well), was added to the lysate, and the plate was incubated for 1 hr at room temp, with shaking. The plate was washed and Substrate Mix was added to the wells. The plate was covered in foil and incubated for 10 min with shaking. Signal in the wells was determined using a plate reader.



