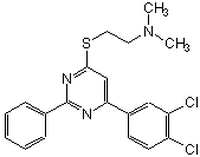
| Description |
A cell-permeable pyrimidine compound that is shown to inhibit the calcineurin (PP2B)-catalyzed dephosphorylation of RII phosphopeptide (Cat. No. 207008), but not pNPP (Cat. Nos. 4876, 487600, 487655, 487663, 487666), in a reversible, calmodulin-independent, and substrate-noncompetitive manner (IC 50 = 3.5 µM; K i = 3.8 µM), while exhibiting little activity against PP1, PP2A, or PP2C even at concentrations as high as 100 µM. Unlike CsA (Cat. No. 239835) and FK506 (Cat. No. 342500), CN585 does not require Cyp A (Cat. No. 239777) and FKBP family immunophilins for PP2B inhibition, nor does CN585 inhibit the peptidyl prolyl cis/trans isomerase (PPIase) activity of Cyp18, FKBP12 (Cat. No. 325902), and Pin1, although binding studies indicate that CN585 and Cyp18/CsA target a common or overlapping binding sites on the catalytic subunit of PP2B. CN585, at 30 µM, is shown to effectively block NFAT nuclear translocation in HeLa cells, NFAT transcription activity in Jurkat cells (IC 50 = 10 µM), as well as IL-2 production in PBMC (IC 50 = 13 µM) upon PMA/ionomycin stimulation.
|