| Description |
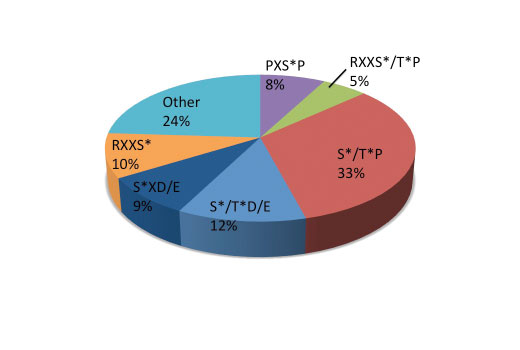
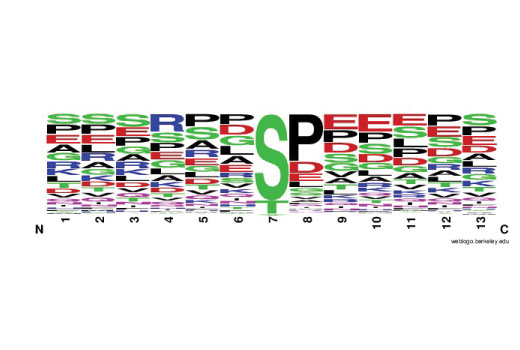
Immobilized metal affinity chromatography, or IMAC, has been widely used to enrich proteins and peptides from biological samples by binding to clusters of negative charge. Divalent transition metal ions Co 2+ , Cu 2+ , Ni 2+ , and Zn 2+ are often used to purify proteins rich in poly-Histidine or Cysteine as well as proteins with metal affinity. Trivalent metal ions, Fe 3+ , Ga 3+ , Al 3+ , as well as Ti 4+ and Zr 4+ are commonly used for phospho-peptide enrichment for proteomic studies (1). Iminodiacetic acid (IDA) or nitrilotriacetic acid (NTA) are used for chelating the metal ions to agarose-coated beads. In comparison studies, NTA has been shown to perform better than IDA at selectively capturing and identifying more phospho-peptides. Ga 3+ and Fe 3+ are comparable with respect to the number of phospho-peptides identified (2). Compared to metal oxide affinity chromatography (MOAC) using TiO 2 , Fe 3+ IMAC performed marginally better with TiO 2 having a preference for acidic phospho-peptides (pI > 4) relative to Fe 3+ which prefered less acidic peptides (pI < 4) (3). The PTMScan ® Fe-NTA Magnetic Beads offer an efficient tool for phospho-peptide enrichment with little or no bias for phospho-residue context. They can be employed independently or in conjunction with immunoaffinity based enrichment to complement any PhosphoScan ® LC-MS/MS proteomic study.
|