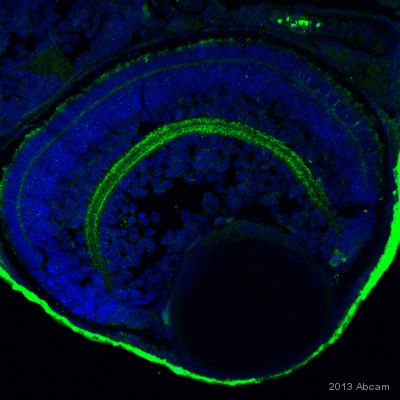
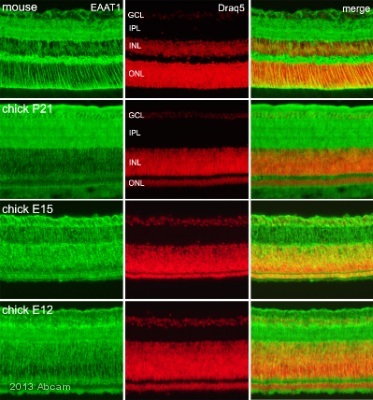
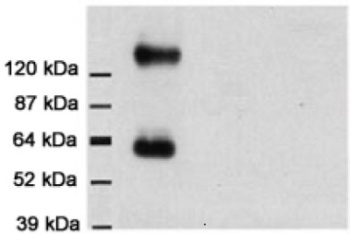
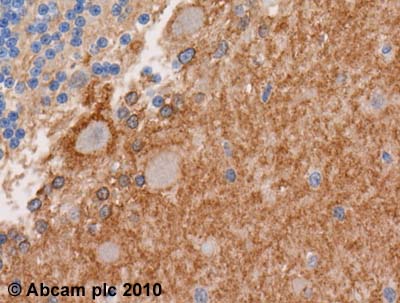
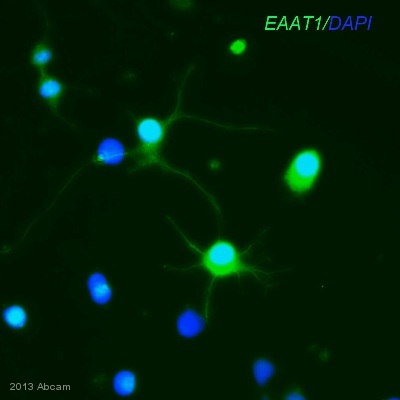
Anti-EAAT1 antibody
| Name | Anti-EAAT1 antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab416 |
| Prices | $401.00 |
| Sizes | 200 µl |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | IHC-F WB ICC/IF FC IHC-P ICC/IF ICC/IF IHC-F |
| Species Reactivities | Mouse, Rat, Human, Zebrafish |
| Antigen | Synthetic peptide, corresponding to 20 residues from the C-terminus of rat EAAT1 (Peptide available as ab127026 |
| Blocking Peptide | EAAT1 peptide |
| Description | Rabbit Polyclonal |
| Gene | SLC1A3 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
In vivo generation of immature inner hair cells in neonatal mouse cochleae by - In vivo generation of immature inner hair cells in neonatal mouse cochleae by
Liu Z, Fang J, Dearman J, Zhang L, Zuo J. PLoS One. 2014 Feb 20;9(2):e89377.
Evidence for aberrant astrocyte hemichannel activity in Juvenile Neuronal Ceroid - Evidence for aberrant astrocyte hemichannel activity in Juvenile Neuronal Ceroid
Burkovetskaya M, Karpuk N, Xiong J, Bosch M, Boska MD, Takeuchi H, Suzumura A, Kielian T. PLoS One. 2014 Apr 15;9(4):e95023.
The db/db mouse: a useful model for the study of diabetic retinal - The db/db mouse: a useful model for the study of diabetic retinal
Bogdanov P, Corraliza L, Villena JA, Carvalho AR, Garcia-Arumi J, Ramos D, Ruberte J, Simo R, Hernandez C. PLoS One. 2014 May 16;9(5):e97302.
Role of Na,K-ATPase alpha1 and alpha2 isoforms in the support of astrocyte - Role of Na,K-ATPase alpha1 and alpha2 isoforms in the support of astrocyte
Illarionova NB, Brismar H, Aperia A, Gunnarson E. PLoS One. 2014 Jun 5;9(6):e98469.
Activation of sodium-dependent glutamate transporters regulates the morphological - Activation of sodium-dependent glutamate transporters regulates the morphological
Martinez-Lozada Z, Waggener CT, Kim K, Zou S, Knapp PE, Hayashi Y, Ortega A, Fuss B. Glia. 2014 Sep;62(9):1543-58.
Neonatal dexamethasone treatment exacerbates hypoxic-ischemic brain injury. - Neonatal dexamethasone treatment exacerbates hypoxic-ischemic brain injury.
Chang KH, Yeh CM, Yeh CY, Huang CC, Hsu KS. Mol Brain. 2013 Apr 18;6:18.
Protection against myocardial ischemia-reperfusion injury at onset of type 2 - Protection against myocardial ischemia-reperfusion injury at onset of type 2
Povlsen JA, Lofgren B, Dalgas C, Birkler RI, Johannsen M, Stottrup NB, Botker HE. PLoS One. 2013 May 21;8(5):e64093.
Repair of astrocytes, blood vessels, and myelin in the injured brain: possible - Repair of astrocytes, blood vessels, and myelin in the injured brain: possible
Jeong HK, Ji KM, Kim J, Jou I, Joe EH. Mol Brain. 2013 Jun 10;6:28.
Regional regulation of glutamate signaling during cuprizone-induced demyelination - Regional regulation of glutamate signaling during cuprizone-induced demyelination
Azami Tameh A, Clarner T, Beyer C, Atlasi MA, Hassanzadeh G, Naderian H. Ann Anat. 2013 Oct;195(5):415-23.
Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic - Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic
Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, Lambert JJ, Belelli D. J Neurosci. 2013 Dec 11;33(50):19534-54.