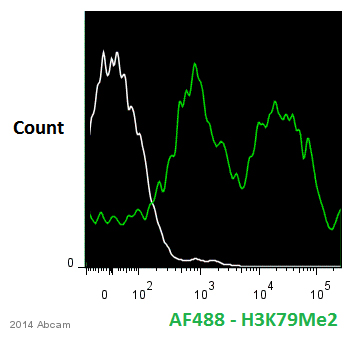
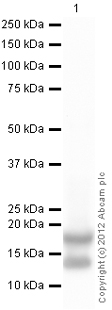
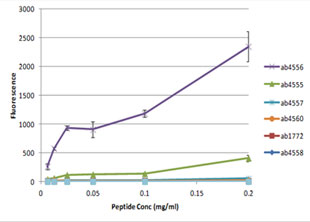
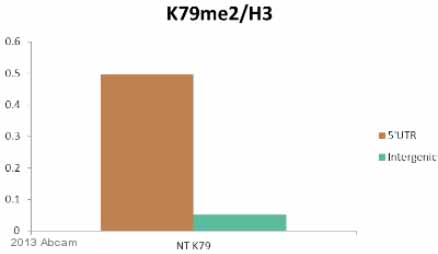
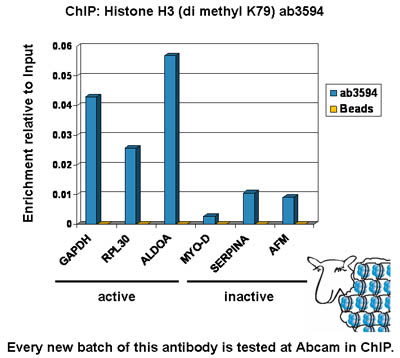
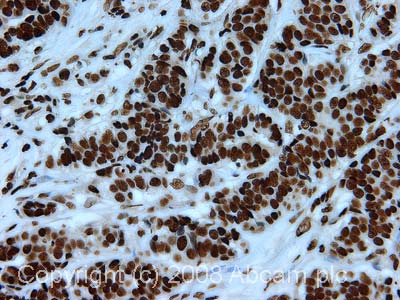
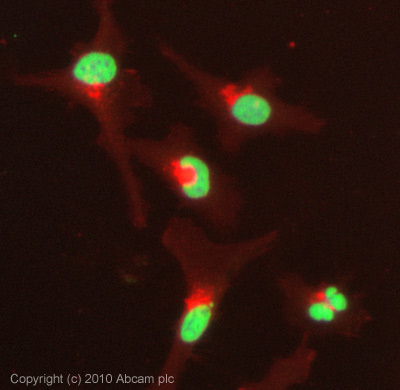
Anti-Histone H3 (di methyl K79) antibody - ChIP Grade
| Name | Anti-Histone H3 (di methyl K79) antibody - ChIP Grade |
|---|---|
| Supplier | Abcam |
| Catalog | ab3594 |
| Prices | $403.00 |
| Sizes | 100 µg |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | ChIP ChIP ChIP ICC/IF ICC/IF WB ChIPseq IHC-P Array |
| Species Reactivities | Mouse, Bovine, Human, Yeast, C. elegans, Broad species, Mammalian, Wheat |
| Antigen | Synthetic peptide conjugated to KLH derived from within residues 50 to the C-terminus of Human Histone H3, di methylated at K79 |
| Blocking Peptide | Human Histone H3 (di methyl K79) peptide |
| Description | Rabbit Polyclonal |
| Gene | HIST3H3 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Initiation of MLL-rearranged AML is dependent on C/EBPalpha. - Initiation of MLL-rearranged AML is dependent on C/EBPalpha.
Ohlsson E, Hasemann MS, Willer A, Lauridsen FK, Rapin N, Jendholm J, Porse BT. J Exp Med. 2014 Jan 13;211(1):5-13.
Mitotic phosphorylation of histone H3 threonine 80. - Mitotic phosphorylation of histone H3 threonine 80.
Hammond SL, Byrum SD, Namjoshi S, Graves HK, Dennehey BK, Tackett AJ, Tyler JK. Cell Cycle. 2014;13(3):440-52.
Effects of RNAi-mediated knockdown of histone methyltransferases on the - Effects of RNAi-mediated knockdown of histone methyltransferases on the
Suzuki MG, Ito H, Aoki F. Int J Mol Sci. 2014 Apr 22;15(4):6772-96.
Uncoupling transcription from covalent histone modification. - Uncoupling transcription from covalent histone modification.
Zhang H, Gao L, Anandhakumar J, Gross DS. PLoS Genet. 2014 Apr 10;10(4):e1004202.
The U4/U6 recycling factor SART3 has histone chaperone activity and associates - The U4/U6 recycling factor SART3 has histone chaperone activity and associates
Long L, Thelen JP, Furgason M, Haj-Yahya M, Brik A, Cheng D, Peng J, Yao T. J Biol Chem. 2014 Mar 28;289(13):8916-30.
DOT1L inhibition sensitizes MLL-rearranged AML to chemotherapy. - DOT1L inhibition sensitizes MLL-rearranged AML to chemotherapy.
Liu W, Deng L, Song Y, Redell M. PLoS One. 2014 May 23;9(5):e98270.
H3K79 methylation directly precedes the histone-to-protamine transition in - H3K79 methylation directly precedes the histone-to-protamine transition in
Dottermusch-Heidel C, Klaus ES, Gonzalez NH, Bhushan S, Meinhardt A, Bergmann M, Renkawitz-Pohl R, Rathke C, Steger K. Andrology. 2014 Sep;2(5):655-65.
Quantitative proteomic discovery of dynamic epigenome changes that control human - Quantitative proteomic discovery of dynamic epigenome changes that control human
O'Connor CM, DiMaggio PA Jr, Shenk T, Garcia BA. Mol Cell Proteomics. 2014 Sep;13(9):2399-410.
Cotranscriptional histone H2B monoubiquitylation is tightly coupled with RNA - Cotranscriptional histone H2B monoubiquitylation is tightly coupled with RNA
Fuchs G, Hollander D, Voichek Y, Ast G, Oren M. Genome Res. 2014 Oct;24(10):1572-83.
Abrogation of MLL-AF10 and CALM-AF10-mediated transformation through genetic - Abrogation of MLL-AF10 and CALM-AF10-mediated transformation through genetic
Chen L, Deshpande AJ, Banka D, Bernt KM, Dias S, Buske C, Olhava EJ, Daigle SR, Richon VM, Pollock RM, Armstrong SA. Leukemia. 2013 Apr;27(4):813-22.