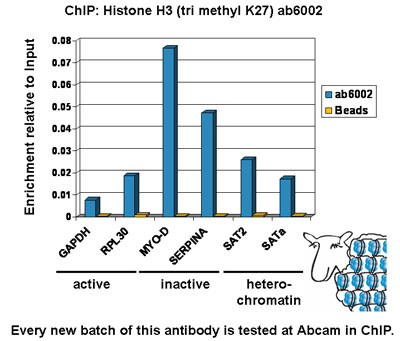
Chromatin was prepared from Hela cells according to the Abcam X-ChIP protocol. Cells were fixed with formaldehyde for 10min. The ChIP was performed with 25µg of chromatin, 5µg of ab6002 (blue), and 20µl of Protein A/G sepharose beads. No antibody was added to the beads control (yellow). The immunoprecipitated DNA was quantified by real time PCR (Taqman approach for active and inactive loci, Sybr green approach for heterochromatic loci). Primers and probes are located in the first kb of the transcribed region.
![All lanes : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/mlLane 1 : Calf Thymus Histone Preparation Nuclear LysateLane 2 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 peptide (ab17163) at 0.5 µg/mlLane 3 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (mono methyl K4) peptide (ab1340) at 0.5 µg/mlLane 4 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (di methyl K4) peptide (ab7768) at 0.5 µg/mlLane 5 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (tri methyl K4) peptide (ab1342) at 0.5 µg/mlLane 6 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (mono methyl K9) peptide (ab1771) at 0.5 µg/mlLane 7 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (di methyl K9) peptide (ab1772) at 0.5 µg/mlLane 8 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (tri methyl K9) peptide (ab1773) at 0.5 µg/m](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/1958_ab6002-238708-WBab60022.jpg)
All lanes : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/mlLane 1 : Calf Thymus Histone Preparation Nuclear LysateLane 2 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 peptide (ab17163) at 0.5 µg/mlLane 3 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (mono methyl K4) peptide (ab1340) at 0.5 µg/mlLane 4 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (di methyl K4) peptide (ab7768) at 0.5 µg/mlLane 5 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (tri methyl K4) peptide (ab1342) at 0.5 µg/mlLane 6 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (mono methyl K9) peptide (ab1771) at 0.5 µg/mlLane 7 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (di methyl K9) peptide (ab1772) at 0.5 µg/mlLane 8 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (tri methyl K9) peptide (ab1773) at 0.5 µg/m
![Lanes 1 - 3 : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/ml (2% BSA)Lanes 4 - 6 : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/ml (3% MILK)Lane 1 : HeLa (Human epithelial carcinoma cell line) Nuclear LysateLane 2 : EED-/- mouse ES Whole Cell LysateLane 3 : WT mouse ES Whole Cell LysateLane 4 : HeLa (Human epithelial carcinoma cell line) Nuclear LysateLane 5 : EED-/- mouse ES Whole Cell LysateLane 6 : WT mouse ES Whole Cell LysateLysates/proteins at 10 µg per lane.SecondaryGoat Anti-Mouse IgG H&L (HRP) preadsorbed (ab97040) at 1/50000 dilutiondeveloped using the ECL techniquePerformed under reducing conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/1959_ab6002-238711-WBab60021.jpg)
Lanes 1 - 3 : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/ml (2% BSA)Lanes 4 - 6 : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/ml (3% MILK)Lane 1 : HeLa (Human epithelial carcinoma cell line) Nuclear LysateLane 2 : EED-/- mouse ES Whole Cell LysateLane 3 : WT mouse ES Whole Cell LysateLane 4 : HeLa (Human epithelial carcinoma cell line) Nuclear LysateLane 5 : EED-/- mouse ES Whole Cell LysateLane 6 : WT mouse ES Whole Cell LysateLysates/proteins at 10 µg per lane.SecondaryGoat Anti-Mouse IgG H&L (HRP) preadsorbed (ab97040) at 1/50000 dilutiondeveloped using the ECL techniquePerformed under reducing conditions.
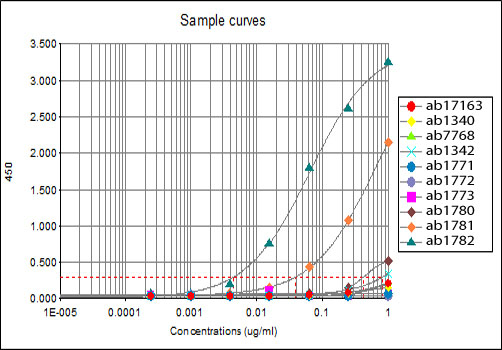
All batches of ab6002 are tested in ELISA against peptides to different Histone H3 modifications. Results show strong binding to Histone H3 - tri methyl K27 immunising peptide (ab1782), indicating that this antibody specifically recognises the Histone H3 - tri methyl K27 modification. Weak binding is also detected against the Histone H3 - di methyl K27 modification (<12%) (ab1781).ab17163 - Histone H3 - unmodifiedab1340 - Histone H3 - mono methyl K4ab7768 - Histone H3 - di methyl K4ab1342 - Histone H3 - tri methyl K4ab1771 - Histone H3 - mono methyl K9ab1772 - Histone H3 - di methyl K9ab1773 - Histone H3 - tri methyl K9ab1780 - Histone H3 - mono methyl K27ab1781 - Histone H3 - di methyl K27ab1782 - Histone H3 - tri methyl K27
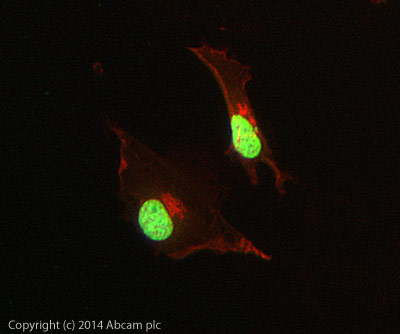
ICC/IF image of ab6002 stained HeLa cells. The cells were 4% PFA fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab6002, 5µg/ml) overnight at +4°C. The secondary antibody (green) was Alexa Fluor® 488 goat anti-mouse IgG (H+L) used at a 1/1000 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM. This antibody also gave a positive result in 4% PFA fixed (10 min) Hek293, HepG2 and MCF7 cells at 5µg/ml, and in 100% methanol fixed (5 min) HeLa, Hek293, HepG2 and MCF7 cells at 5µg/ml.
![All lanes : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/mlLane 1 : Calf Thymus Histone Preparation Nuclear Lysate at 0.5 µg with BSA BLOCKLane 2 : Calf Thymus Histone Preparation Nuclear Lysate at 0.5 µg with MILK BLOCKLane 3 : MEF1 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with BSA BLOCKLane 4 : NIH 3T3 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with BSA BLOCKLane 5 : MEF1 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with MILK BLOCKLane 6 : NIH 3T3 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with MILK BLOCKSecondaryGoat polyclonal to Mouse IgG - H&L - Pre-Adsorbed (HRP) at 1/3000 dilutionPerformed under reducing conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/1962_Histone-H3-Primary-antibodies-ab6002-24.jpg)
All lanes : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/mlLane 1 : Calf Thymus Histone Preparation Nuclear Lysate at 0.5 µg with BSA BLOCKLane 2 : Calf Thymus Histone Preparation Nuclear Lysate at 0.5 µg with MILK BLOCKLane 3 : MEF1 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with BSA BLOCKLane 4 : NIH 3T3 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with BSA BLOCKLane 5 : MEF1 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with MILK BLOCKLane 6 : NIH 3T3 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with MILK BLOCKSecondaryGoat polyclonal to Mouse IgG - H&L - Pre-Adsorbed (HRP) at 1/3000 dilutionPerformed under reducing conditions.
![Immunofluorescent imaging of human cells (U2OS) with ab6002 reveals broadly dispersed interphase nuclear staining corresponding to trimethylation of K27, with multiple foci of brighter staining, exactly agreeing with published studies of K27-trimethyl IF [See figure 3 of Peters et al Mol Cell 12(6):1577-1589 (2003)] . The lack of nucleolar or cytoplasmic staining background confirms the high specificity of the antibody in this application. IF was performed with a standard paraformaldehyde technique (fixed in PBS buffered PFH 4% for 5 minutes, permeabilised with 0.5% triton-PBS for 5 minutes, blocked with 5% milk / 0.2% tween for one hour. Primary antibody used at 1/200 in 5% milk / 0.2% TWEEN for one hour, secondary antibody Alexa 488 for 30 minutes. All blocking and incubation steps carried out at 37 degrees C.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/1963_ab6002_4.jpg)
Immunofluorescent imaging of human cells (U2OS) with ab6002 reveals broadly dispersed interphase nuclear staining corresponding to trimethylation of K27, with multiple foci of brighter staining, exactly agreeing with published studies of K27-trimethyl IF [See figure 3 of Peters et al Mol Cell 12(6):1577-1589 (2003)] . The lack of nucleolar or cytoplasmic staining background confirms the high specificity of the antibody in this application. IF was performed with a standard paraformaldehyde technique (fixed in PBS buffered PFH 4% for 5 minutes, permeabilised with 0.5% triton-PBS for 5 minutes, blocked with 5% milk / 0.2% tween for one hour. Primary antibody used at 1/200 in 5% milk / 0.2% TWEEN for one hour, secondary antibody Alexa 488 for 30 minutes. All blocking and incubation steps carried out at 37 degrees C.
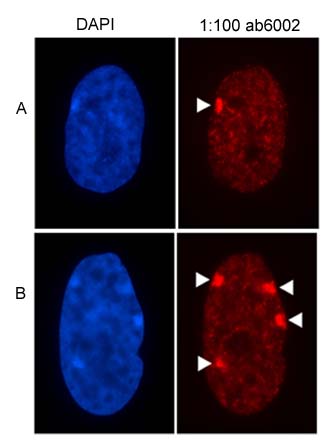
Figure showing the nuclear distribution of H3 (tri-methyl K27) antibody, ab6002 in a) a 46 chromosome, XX cell line, and b) a 49 chromosome, XXXXX cell line. The location of facultative heterochromatin at the inactive X chromosome is indicated by white arrow heads.
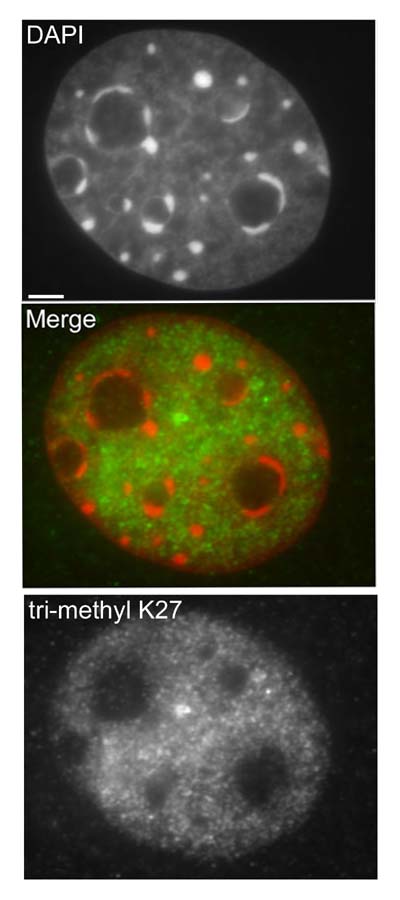
Interphase 10T1/2 mouse fibroblasts were paraformaldehyde fixed (4%), immunofluorescently labeled with anti-trimethyl K27 antibody (ab6002) and counterstained with DAPI. The merge image presents the DAPI and ab6002 channels as red and green, respectively. The scale bar represents 3µm.
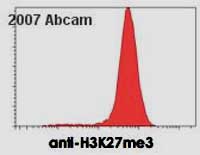
ab6002 staining mouse embyronic stem cells by flow cytometry. The ES cell colonies were trypsinized and permeabilized prior to blocking and staining with the antibody (1ug/1.5 x 10 5 cells. A Cy3 conjugated anti-mouse antibody was used as the secondary.See Abreview
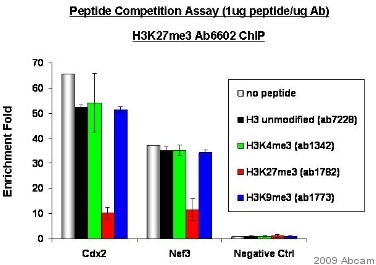
Chromatin was prepared from nuclear lysate of the mouse embryonic stem cells. The cross-linking (X-ChiP) technique was used, crosslinking was performed for 15 minutes in 1% formaldehyde. The primary antibody was diluted to 0.0133µg/µg chromatin and incubated in ChIP Sonication Buffer with the sample for 24 hours at 4°C. The immunoprecipitated DNA was quantified by real time PCR.Cdx2: PCR primers situated in the promoter regions of Caudal type homeobox transcription factor 2Nef3: PCR primers situated in the promoter regions of neurofilament 3See Abreview
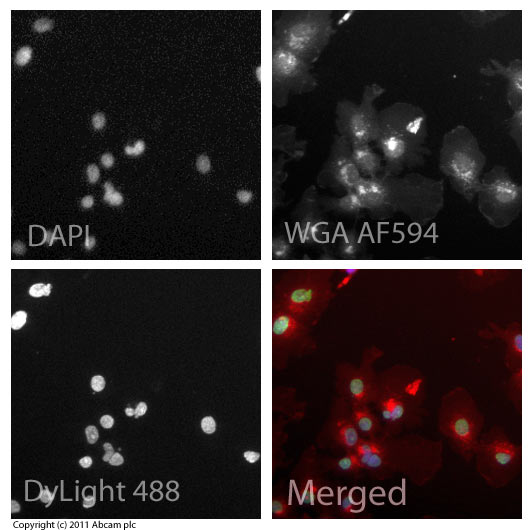
ICC/IF image of ab6002 stained HepG2 cells. The cells were 100% methanol fixed (5 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab6002, 5µg/ml) overnight at +4°C. The secondary antibody (green) was ab96879, goat anti-mouse DyLight® 488 (IgG H+L) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.

![All lanes : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/mlLane 1 : Calf Thymus Histone Preparation Nuclear LysateLane 2 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 peptide (ab17163) at 0.5 µg/mlLane 3 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (mono methyl K4) peptide (ab1340) at 0.5 µg/mlLane 4 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (di methyl K4) peptide (ab7768) at 0.5 µg/mlLane 5 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (tri methyl K4) peptide (ab1342) at 0.5 µg/mlLane 6 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (mono methyl K9) peptide (ab1771) at 0.5 µg/mlLane 7 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (di methyl K9) peptide (ab1772) at 0.5 µg/mlLane 8 : Calf Thymus Histone Preparation Nuclear Lysate with Human Histone H3 (tri methyl K9) peptide (ab1773) at 0.5 µg/m](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/1958_ab6002-238708-WBab60022.jpg)
![Lanes 1 - 3 : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/ml (2% BSA)Lanes 4 - 6 : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/ml (3% MILK)Lane 1 : HeLa (Human epithelial carcinoma cell line) Nuclear LysateLane 2 : EED-/- mouse ES Whole Cell LysateLane 3 : WT mouse ES Whole Cell LysateLane 4 : HeLa (Human epithelial carcinoma cell line) Nuclear LysateLane 5 : EED-/- mouse ES Whole Cell LysateLane 6 : WT mouse ES Whole Cell LysateLysates/proteins at 10 µg per lane.SecondaryGoat Anti-Mouse IgG H&L (HRP) preadsorbed (ab97040) at 1/50000 dilutiondeveloped using the ECL techniquePerformed under reducing conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/1959_ab6002-238711-WBab60021.jpg)


![All lanes : Anti-Histone H3 (tri methyl K27) antibody [mAbcam 6002] - ChIP Grade (ab6002) at 1 µg/mlLane 1 : Calf Thymus Histone Preparation Nuclear Lysate at 0.5 µg with BSA BLOCKLane 2 : Calf Thymus Histone Preparation Nuclear Lysate at 0.5 µg with MILK BLOCKLane 3 : MEF1 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with BSA BLOCKLane 4 : NIH 3T3 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with BSA BLOCKLane 5 : MEF1 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with MILK BLOCKLane 6 : NIH 3T3 (Mouse embryonic fibroblast cell line) Whole Cell Lysate at 10 µg with MILK BLOCKSecondaryGoat polyclonal to Mouse IgG - H&L - Pre-Adsorbed (HRP) at 1/3000 dilutionPerformed under reducing conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/1962_Histone-H3-Primary-antibodies-ab6002-24.jpg)
![Immunofluorescent imaging of human cells (U2OS) with ab6002 reveals broadly dispersed interphase nuclear staining corresponding to trimethylation of K27, with multiple foci of brighter staining, exactly agreeing with published studies of K27-trimethyl IF [See figure 3 of Peters et al Mol Cell 12(6):1577-1589 (2003)] . The lack of nucleolar or cytoplasmic staining background confirms the high specificity of the antibody in this application. IF was performed with a standard paraformaldehyde technique (fixed in PBS buffered PFH 4% for 5 minutes, permeabilised with 0.5% triton-PBS for 5 minutes, blocked with 5% milk / 0.2% tween for one hour. Primary antibody used at 1/200 in 5% milk / 0.2% TWEEN for one hour, secondary antibody Alexa 488 for 30 minutes. All blocking and incubation steps carried out at 37 degrees C.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/1963_ab6002_4.jpg)




