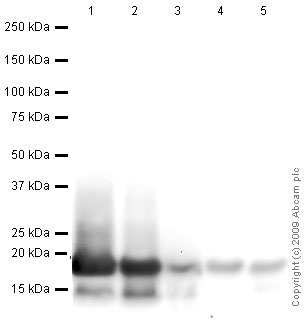
All lanes : Anti-Histone H3 antibody - Nuclear Loading Control and ChIP Grade (ab1791) at 1 µg/mlLane 1 : HeLa (Human epithelial carcinoma cell line) Whole Cell LysateLane 2 : NIH 3T3 (Mouse embryonic fibroblast cell line) Whole Cell Lysate (ab7179)Lane 3 : Drosophila embryo nuclear extract (from melanogaster embryos 0-12Hr)Lane 4 : S.cerevisiae (Y190) Whole Cell Lysate Lane 5 : S.pombe Whole Cell Lysate Lysates/proteins at 10 µg per lane.SecondaryGoat polyclonal to Rabbit IgG - H&L - Pre-Adsorbed (HRP) at 1/3000 dilutionPerformed under reducing conditions.
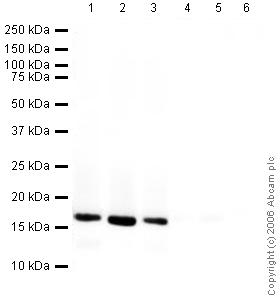
All lanes : Anti-Histone H3 antibody - Nuclear Loading Control and ChIP Grade (ab1791) at 1/1000 dilutionLane 1 : A431 (Human epithelial carcinoma cell line) Whole Cell LysateLane 2 : Jurkat (Human T cell lymphoblast-like cell line) Whole Cell LysateLane 3 : HEK293 (Human embryonic kidney cell line) Whole Cell LysateLane 4 : A431 (Human epithelial carcinoma cell line) Whole Cell Lysate with Human Histone H3 peptide (ab12149) at 1 µg/mlLane 5 : Jurkat (Human T cell lymphoblast-like cell line) Whole Cell Lysate with Human Histone H3 peptide (ab12149) at 1 µg/mlLane 6 : HEK293 (Human embryonic kidney cell line) Whole Cell Lysate with Human Histone H3 peptide (ab12149) at 1 µg/mlLysates/proteins at 20 µg per lane.SecondaryGoat Anti-Rabbit IgG H&L (HRP) (ab6721) at 1/5000 dilutiondeveloped using the ECL techniquePerformed under reducing conditions.
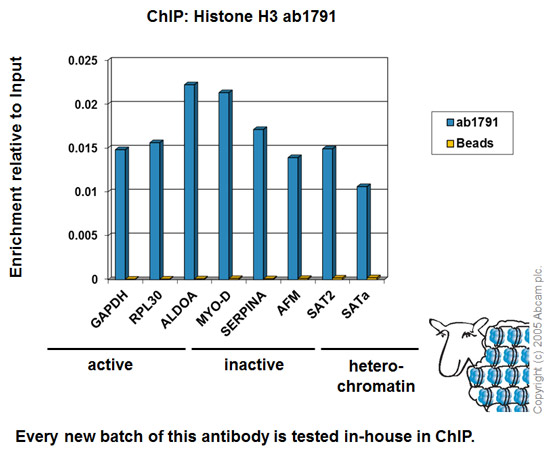
Chromatin was prepared from HeLa cells according to the Abcam X-ChIP protocol. Cells were fixed with formaldehyde for 10 minutes. The ChIP was performed with 25µg of chromatin, 2µg of ab1791 (blue), and 20µl of Protein A/G sepharose beads. No antibody was added to the beads control (yellow). The immunoprecipitated DNA was quantified by real time PCR (Taqman approach for active and inactive loci, Sybr green approach for heterochromatic loci). Primers and probes are located in the first kb of the transcribed region.
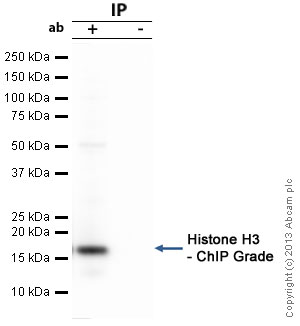
Histone H3 - ChIP Grade was immunoprecipitated using 0.5mg HeLa whole cell extract, 5µg of Rabbit polyclonal to and 50µl of protein G magnetic beads (+). No antibody was added to the control (-).The antibody was incubated under agitation with Protein G beads for 10min, HeLa whole cell extract lysate diluted in RIPA buffer was added to each sample and incubated for a further 10min under agitation.Proteins were eluted by addition of 40µl SDS loading buffer and incubated for 10min at 70°C; 10µl of each sample was separated on a SDS PAGE gel, transferred to a nitrocellulose membrane, blocked with 5% BSA and probed with ab1791.Secondary Antibody: Mouse anti-rabbit HRP light chain (HRP) (ab99697).Band: 15kDa; Histone H3 - ChIP Grade

Hela cells cultured on coverslips were fixed with 4% paraformaldehyde and then stained with ab1791 (green) at a working dilution of 1/500. The DNA stained with DAPI is shown in red. (100x magnification).
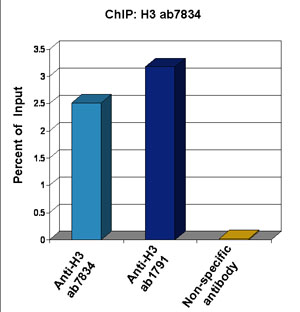
Chromatin from Xenopus laevis oocytes was prepared according to the Abcam X-ChIP protocol. Oocytes were fixed with formaldehyde for 10 min. The ChIP was performed with 25 mg of chromatin, 3 mg of ab7834 (anti-H3, light blue) and 3 µg of ab1791 (anti-H3, dark blue), and 20 ml of Protein A/G sepharose beads. A non-specific antibody was used as a control (yellow). The immunoprecipitated DNA was quantified by real time PCR (Taqman approach).
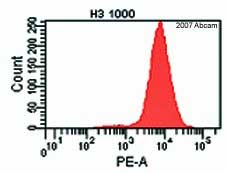
ab1791 staining mouse embryonic stem cells by flow cytometry (gated on all living cells). The cell colonies were trypsinized and incubated with the antibody 1ug/1.5 x 105cells in a permeabilization buffer. A PE conjugated goat anti-rabbit antibody was used as the secondary.See Abreview
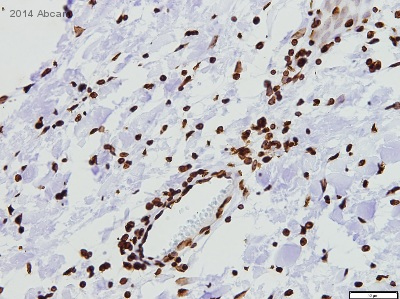
ab1791 staining Histone H3 in Human infantile fibromatosis tissue sections by Immunohistochemistry (IHC-P - paraformaldehyde-fixed, paraffin-embedded sections). Tissue was fixed with formaldehyde and blocked with 1% FBS/BSA for 3 hours at room temperature; antigen retrieval was by heat mediation in Tris pH9. Samples were incubated with primary antibody (1/100 in TBS + 1% BSA + 1% FBS) for 16 hours. An undiluted HRP-conjugated goat anti-rabbit IgG polyclonal was used as the secondary antibody.See Abreview
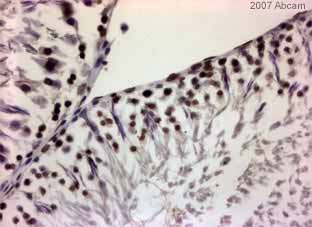
ab1791 staining rat testes tissue sections by IHC-P. Sections were formaldehyde fixed and subjected to heat mediated antigen retrieval prior to blocking with 5% serum for 30 minutes at 22°C. The primary antibody was diluted 1/400 and incubated with the sample for 30 minutes at 22°C. A HRP-conjugated goat anti-rabbit antibody diluted 1/400 was used as the secondary.See Abreview
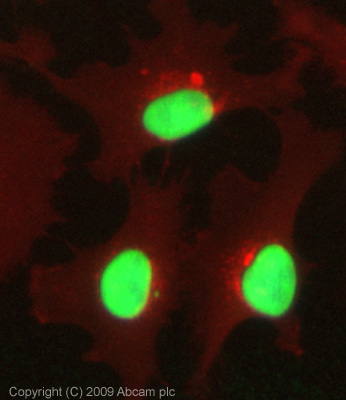
ICC/IF image of ab1791 stained HeLa cells. The cells were 100% methanol fixed (5 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab1791, 1µg/ml) overnight at +4°C. The secondary antibody (green) was Alexa Fluor® 488 goat anti-rabbit IgG (H+L) used at a 1/1000 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM. This antibody also gave a positive result in 5% PFA fixed (5 min) Hek293, HepG2 and MCF7 cells at 1µg/ml, and in 4% PFA fixed (10 min) HeLa and Hek293 cells at 1µg/ml.
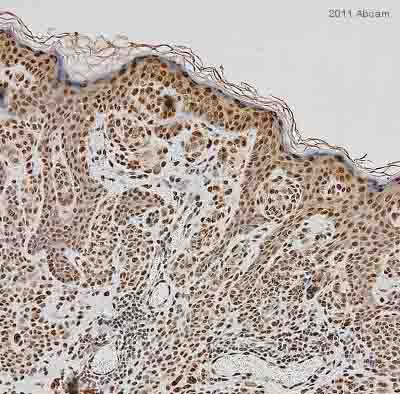
ab1791 staining Histone H3 in human benign nevi tissue by Immunohistochemistry (Formalin/PFA-fixed paraffin-embedded sections). Tissue was fixed with formaldehyde and a heat mediated antigen retrieval step was performed. Samples were then blocked with 2.5% normal horse serum for 20 minutes followed by incubation with the primary antibody at a 1/200 dilution for 16 hours at 4°C. An undiluted HRP-conjugated rabbit polyclonal was used as the secondary antibody.See Abreview
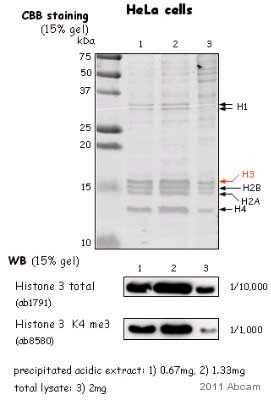
All lanes : Anti-Histone H3 antibody - Nuclear Loading Control and ChIP Grade (ab1791) at 1/10000 dilutionLane 1 : HeLa nuclear lysateLane 2 : HeLa nuclear lysateLane 3 : HeLa nuclear lysateLysates/proteins at 2 µg per lane.SecondaryHRP conjugated donkey polyclonal at 1/2000 dilutiondeveloped using the ECL technique
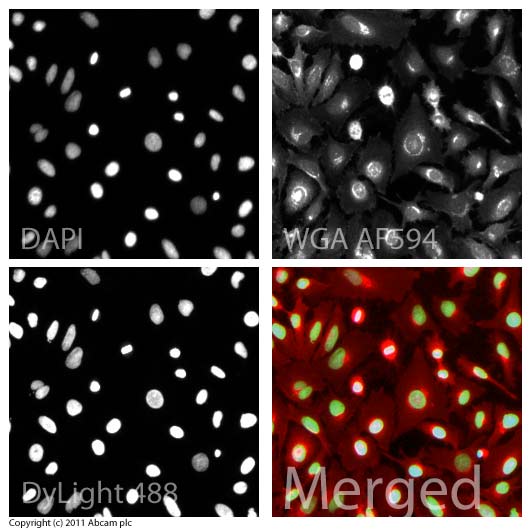
ICC/IF image of ab1791 stained HeLa cells. The cells were 4% formaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab1791, 1µg/ml) overnight at +4°C. The secondary antibody (green) was ab96899, goat anti-rabbit DyLight® 488 IgG (H+L) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.
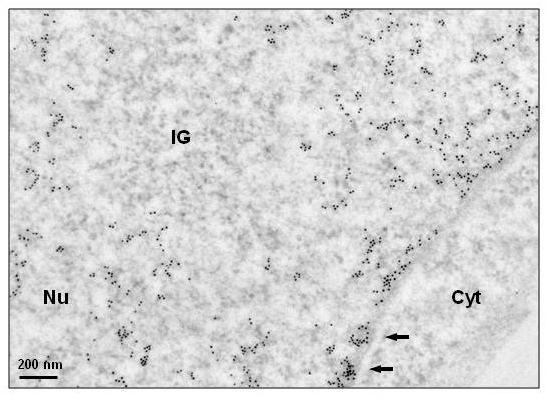
Histone H3 immunogold detection in HeLa cells.Ulltra-thin sections of paraformaldehyde-fixed, Lowicryl K4M embedded cells were incubated sequentially with ab1791 antibody and an anti-rabbit antibody coupled to 10 nm gold particles. Notice absence of chromatin within Interchromatin Granules (IG), a reservoir of spliceosomal snRNPs, and high labeling of patches of condensed chromatin close to the nuclear envelope (arrows). Nu: nucleus, Cyt: cytoplasm. Image courtesy of Gerard Pierron, IGR-Villejuif, France. Sample : humanType : HeLa cellsFixative : either paraformaldehyde 4% or 1.6% glutaraldehyde in 0.1M Millonig’s buffer.Embedding : in Lowicryl 4KM at -20°C under UV.Ultrathin-sections deposited on formvar-coated carbonated EM-grids.Blocking step: 5% BSA for 30 seconds at RT.Primary antibody: Ab1791 diluted 1/50 in PBS, for 1h at RT.Secondary antibody: BBI International anti-rabbit