Anti-HMG14 antibody - ChIP Grade
| Name | Anti-HMG14 antibody - ChIP Grade |
|---|---|
| Supplier | Abcam |
| Catalog | ab5212 |
| Prices | $401.00 |
| Sizes | 100 µg |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | ICC/IF ICC/IF ChIP ICC/IF IP IHC-P FC WB |
| Species Reactivities | Mouse, Rat, Human, Bovine |
| Antigen | Synthetic peptide derived from residues 50 to the C-terminus of Human HMGN1/ HMG14 |
| Blocking Peptide | Human HMG14 peptide |
| Description | Rabbit Polyclonal |
| Gene | HMGN1 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
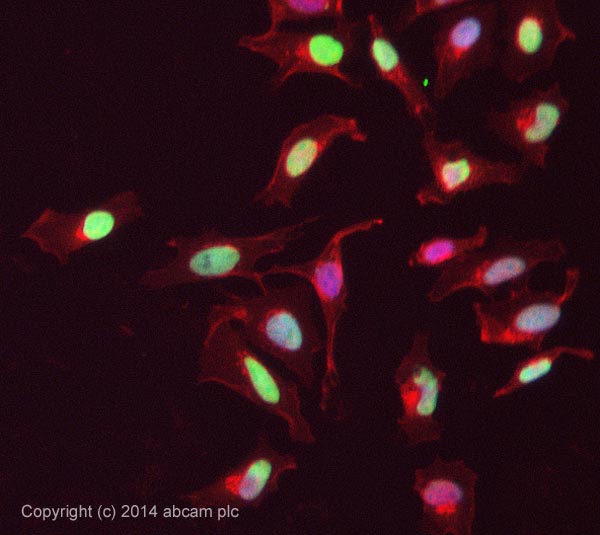
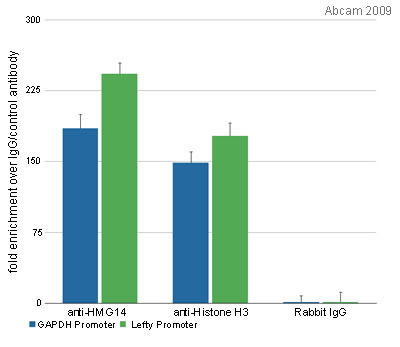
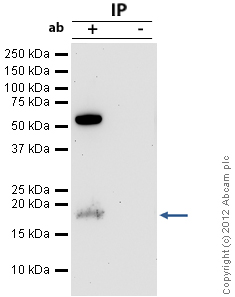
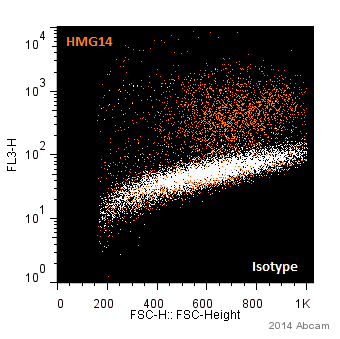
Product images
Product References
Triplication of a 21q22 region contributes to B cell transformation through HMGN1 - Triplication of a 21q22 region contributes to B cell transformation through HMGN1
Lane AA, Chapuy B, Lin CY, Tivey T, Li H, Townsend EC, van Bodegom D, Day TA, Wu SC, Liu H, Yoda A, Alexe G, Schinzel AC, Sullivan TJ, Malinge S, Taylor JE, Stegmaier K, Jaffe JD, Bustin M, te Kronnie G, Izraeli S, Harris MH, Stevenson KE, Neuberg D, Silverman LB, Sallan SE, Bradner JE, Hahn WC, Crispino JD, Pellman D, Weinstock DM. Nat Genet. 2014 Jun;46(6):618-23.
Reprogramming ovarian and breast cancer cells into non-cancerous cells by - Reprogramming ovarian and breast cancer cells into non-cancerous cells by
Hu T, Chung YM, Guan M, Ma M, Ma J, Berek JS, Hu MC. Sci Rep. 2014 Jul 24;4:5810.
Transcription-dependent dynamic supercoiling is a short-range genomic force. - Transcription-dependent dynamic supercoiling is a short-range genomic force.
Kouzine F, Gupta A, Baranello L, Wojtowicz D, Ben-Aissa K, Liu J, Przytycka TM, Levens D. Nat Struct Mol Biol. 2013 Mar;20(3):396-403.
UV-sensitive syndrome protein UVSSA recruits USP7 to regulate - UV-sensitive syndrome protein UVSSA recruits USP7 to regulate
Schwertman P, Lagarou A, Dekkers DH, Raams A, van der Hoek AC, Laffeber C, Hoeijmakers JH, Demmers JA, Fousteri M, Vermeulen W, Marteijn JA. Nat Genet. 2012 May;44(5):598-602.
Identification of nuclear protein targets for six leukemogenic tyrosine kinases - Identification of nuclear protein targets for six leukemogenic tyrosine kinases
Pierce A, Williamson A, Jaworska E, Griffiths JR, Taylor S, Walker M, Aspinall-O'Dea M, Spooncer E, Unwin RD, Poolman T, Ray D, Whetton AD. PLoS One. 2012;7(6):e38928.
FOXO3 signalling links ATM to the p53 apoptotic pathway following DNA damage. - FOXO3 signalling links ATM to the p53 apoptotic pathway following DNA damage.
Chung YM, Park SH, Tsai WB, Wang SY, Ikeda MA, Berek JS, Chen DJ, Hu MC. Nat Commun. 2012;3:1000.
Dynamic changes in histone H3 phosphoacetylation during early embryonic stem cell - Dynamic changes in histone H3 phosphoacetylation during early embryonic stem cell
Lee ER, McCool KW, Murdoch FE, Fritsch MK. J Biol Chem. 2006 Jul 28;281(30):21162-72. Epub 2006 May 25.