Anti-LAMP1 antibody [H4A3]
| Name | Anti-LAMP1 antibody [H4A3] |
|---|---|
| Supplier | Abcam |
| Catalog | ab25630 |
| Prices | $400.00 |
| Sizes | 100 µg |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG1 |
| Clone | H4A3 |
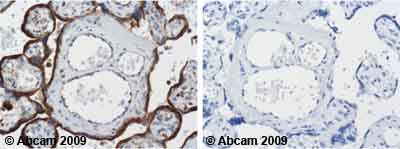
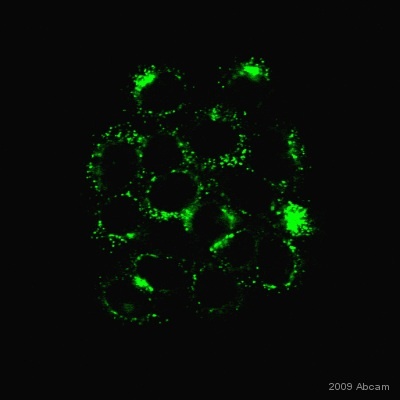
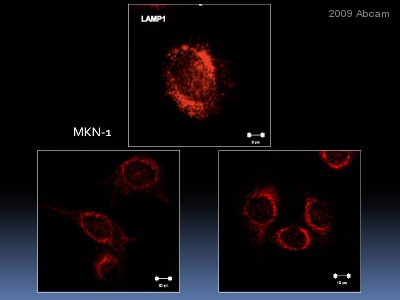
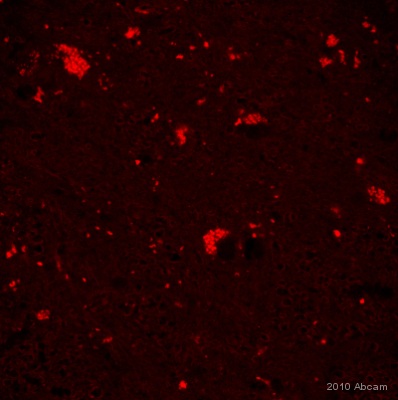
| Applications | ICC/IF ICC/IF FC IHC-F WB IHC-P |
| Species Reactivities | Rat, Human, Monkey |
| Antigen | The details of the immunogen for this antibody are not available |
| Description | Mouse Monoclonal |
| Gene | LAMP1 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Cellular mechanisms of toll-like receptor-3 activation in the thalamus are - Cellular mechanisms of toll-like receptor-3 activation in the thalamus are
Vontell R, Supramaniam V, Wyatt-Ashmead J, Gressens P, Rutherford M, Hagberg H, Thornton C. J Neuropathol Exp Neurol. 2015 Mar;74(3):273-85. doi:
QSOX1 inhibits autophagic flux in breast cancer cells. - QSOX1 inhibits autophagic flux in breast cancer cells.
Poillet L, Pernodet N, Boyer-Guittaut M, Adami P, Borg C, Jouvenot M, Delage-Mourroux R, Despouy G. PLoS One. 2014 Jan 24;9(1):e86641.
Filamin A promotes dynamin-dependent internalization of - Filamin A promotes dynamin-dependent internalization of
Noam Y, Ehrengruber MU, Koh A, Feyen P, Manders EM, Abbott GW, Wadman WJ, Baram TZ. J Biol Chem. 2014 Feb 28;289(9):5889-903.
Anti-inflammatory response following uptake of apoptotic bodies by meningothelial - Anti-inflammatory response following uptake of apoptotic bodies by meningothelial
Li J, Fang L, Meyer P, Killer HE, Flammer J, Neutzner A. J Neuroinflammation. 2014 Feb 24;11:35.
Persistence of Coxiella burnetii, the agent of Q fever, in murine adipose tissue. - Persistence of Coxiella burnetii, the agent of Q fever, in murine adipose tissue.
Bechah Y, Verneau J, Ben Amara A, Barry AO, Lepolard C, Achard V, Panicot-Dubois L, Textoris J, Capo C, Ghigo E, Mege JL. PLoS One. 2014 May 16;9(5):e97503.
Host-derived, pore-forming toxin-like protein and trefoil factor complex protects - Host-derived, pore-forming toxin-like protein and trefoil factor complex protects
Xiang Y, Yan C, Guo X, Zhou K, Li S, Gao Q, Wang X, Zhao F, Liu J, Lee WH, Zhang Y. Proc Natl Acad Sci U S A. 2014 May 6;111(18):6702-7. doi:
Glutamine deprivation stimulates mTOR-JNK-dependent chemokine secretion. - Glutamine deprivation stimulates mTOR-JNK-dependent chemokine secretion.
Shanware NP, Bray K, Eng CH, Wang F, Follettie M, Myers J, Fantin VR, Abraham RT. Nat Commun. 2014 Sep 25;5:4900.
TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein - TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein
Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. Proc Natl Acad Sci U S A. 2013 May 7;110(19):E1817-26. doi:
WNK4 inhibits plasma membrane targeting of NCC through regulation of syntaxin13 - WNK4 inhibits plasma membrane targeting of NCC through regulation of syntaxin13
Chung WY, Park HW, Han JW, Lee MG, Kim JY. Cell Signal. 2013 Dec;25(12):2469-77.
Mitochondria localize to the cleavage furrow in mammalian cytokinesis. - Mitochondria localize to the cleavage furrow in mammalian cytokinesis.
Lawrence EJ, Mandato CA. PLoS One. 2013 Aug 21;8(8):e72886.
![Overlay histogram showing Jurkat cells stained with ab25630 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab25630, 1/20 dilution) for 30 min at 22°C. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22°C. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed. This antibody gave a positive signal in Jurkat cells fixed with methanol (5 min)/permeabilized in 0.1% PBS-Tween used under the same conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/14972_LAMP1-Primary-antibodies-ab25630-14.jpg)

![All lanes : Anti-LAMP1 antibody [H4A3] (ab25630) at 1/10000 dilutionLane 1 : Iron treated 3 month old liver at 20 µgLane 2 : Untreated 3 month old liver at 20 µgLane 3 : One month old untreated liverdeveloped using the ECL techniquePerformed under reducing conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/14978_LAMP1-Primary-antibodies-ab25630-13.jpg)