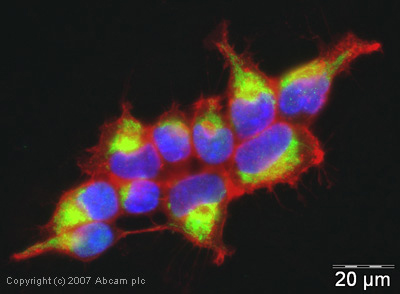
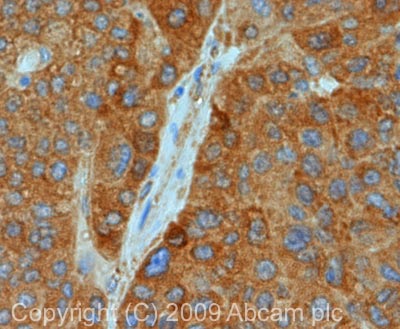
Anti-MAVS antibody
| Name | Anti-MAVS antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab25084 |
| Prices | $390.00 |
| Sizes | 100 µg |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | IP WB ICC/IF ICC/IF IHC-P |
| Species Reactivities | Human, Bovine |
| Antigen | Synthetic peptide conjugated to KLH derived from within residues 150 - 250 of Human MAVS |
| Description | Rabbit Polyclonal |
| Gene | MAVS |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize - Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize
Lifland AW, Jung J, Alonas E, Zurla C, Crowe JE Jr, Santangelo PJ. J Virol. 2012 Aug;86(15):8245-58.
TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of - TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of
Matsushima-Miyagi T, Hatano K, Nomura M, Li-Wen L, Nishikawa T, Saga K, Shimbo T, Kaneda Y. Clin Cancer Res. 2012 Nov 15;18(22):6271-83.
NLRX1 is a regulator of mitochondrial antiviral immunity. - NLRX1 is a regulator of mitochondrial antiviral immunity.
Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, Lich JD, Heise MT, Chen Z, Ting JP. Nature. 2008 Jan 31;451(7178):573-7.
Hepatitis A virus protein 2B suppresses beta interferon (IFN) gene transcription - Hepatitis A virus protein 2B suppresses beta interferon (IFN) gene transcription
Paulmann D, Magulski T, Schwarz R, Heitmann L, Flehmig B, Vallbracht A, Dotzauer A. J Gen Virol. 2008 Jul;89(Pt 7):1593-604.
The mitochondrial antiviral signaling protein, MAVS, is cleaved during apoptosis. - The mitochondrial antiviral signaling protein, MAVS, is cleaved during apoptosis.
Scott I, Norris KL. Biochem Biophys Res Commun. 2008 Oct 10;375(1):101-6. doi:
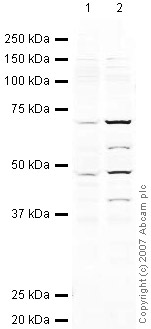
![MAVS was immunoprecipitated using 0.5mg Jurkat whole cell extract, 5µg of Rabbit polyclonal to MAVS and 50µl of protein G magnetic beads (+). No antibody was added to the control (-). The antibody was incubated under agitation with Protein G beads for 10min, Jurkat whole cell extract lysate diluted in RIPA buffer was added to each sample and incubated for a further 10min under agitation.Proteins were eluted by addition of 40µl SDS loading buffer and incubated for 10min at 70oC; 10µl of each sample was separated on a SDS PAGE gel, transferred to a nitrocellulose membrane, blocked with 5% BSA and probed with ab25084.Secondary: Mouse monoclonal [SB62a] Secondary Antibody to Rabbit IgG light chain (HRP) (ab99697).Band: 70kDa: MAVS](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/19952_MAVS-Primary-antibodies-ab25084-6.jpg)