![ab190565 staining NeuN in NGF-differentiated PC12 cells (7 days). The cells were fixed with 100% methanol (5min) and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated with ab190565 at 1/50 dilution (shown in red) and ab7291 (Mouse monoclonal [DM1A] to alpha Tubulin) at 1µg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an Alexa Fluor® 488 Goat anti-Mouse secondary (ab150117) at 2 μg/ml (shown in green). Nuclear DNA was labelled in blue with DAPI.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/29212_ab190565-228185-ab190565AP200570410ugMeOHPC127d.jpg)
ab190565 staining NeuN in NGF-differentiated PC12 cells (7 days). The cells were fixed with 100% methanol (5min) and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated with ab190565 at 1/50 dilution (shown in red) and ab7291 (Mouse monoclonal [DM1A] to alpha Tubulin) at 1µg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an Alexa Fluor® 488 Goat anti-Mouse secondary (ab150117) at 2 μg/ml (shown in green). Nuclear DNA was labelled in blue with DAPI.
![ab190565 staining NeuN in U87-MG cells. The cells were fixed with 100% methanol (5min) and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated with ab190565 at 1/50 dilution(shown in red) and ab7291 (Mouse monoclonal [DM1A] to alpha Tubulin) at 1µg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an Alexa Fluor® 488 Goat anti-Mouse secondary (ab150117) at 2 μg/ml (shown in green). Nuclear DNA was labelled in blue with DAPI.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/29213_ab190565-230909-Layoutab190565directlyla.jpg)
ab190565 staining NeuN in U87-MG cells. The cells were fixed with 100% methanol (5min) and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated with ab190565 at 1/50 dilution(shown in red) and ab7291 (Mouse monoclonal [DM1A] to alpha Tubulin) at 1µg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an Alexa Fluor® 488 Goat anti-Mouse secondary (ab150117) at 2 μg/ml (shown in green). Nuclear DNA was labelled in blue with DAPI.
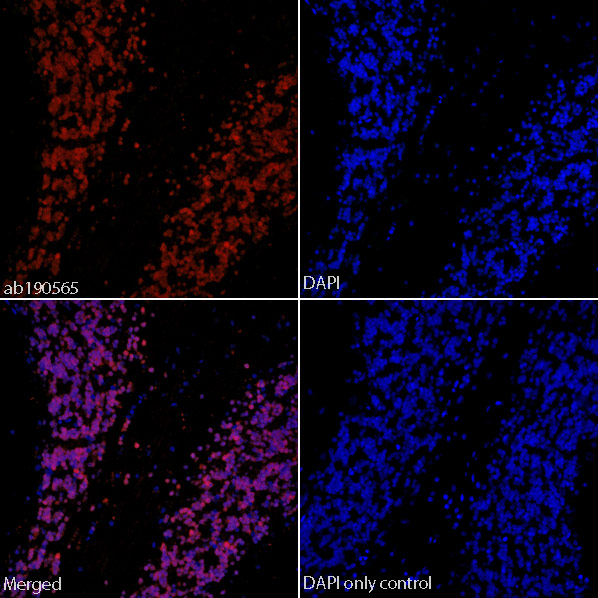
IHC image of ab190565 staining in formalin fixed paraffin embedded tissue section of normal human cerebellum.The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH6) in a Dako Pascal pressure cooker using the standard factory-set regime. Non-specific protein-protein interactions were then blocked using in TBS containing 0.025% (v/v) Triton X-100, 0.3M (w/v) glycine and 3% (w/v) BSA for 1h at room temperature. The section was then incubated with ab190565 (1/50) in TBS containing 0.025% (v/v) Triton X-100 and 3% (w/v) BSA overnight at +4°C. The section was then counterstained and mounted with SlowFade® Gold Antifade Mountant with DAPI.The DAPI only control (no antibody) inset shows no autoflourescence, demonstrating that any Alexa Fluor® 647 signal is dervied directly from bound ab190565. The separate images of ab190565 and DAPI alone, combined with the merged version of both signals, shows predominant co-localisation of the Alexa Fluor® 647 sign

IHC image of ab190565 staining in formalin fixed paraffin embedded tissue section of normal rat cerebellum.The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH6) in a Dako Pascal pressure cooker using the standard factory-set regime. Non-specific protein-protein interactions were then blocked using in TBS containing 0.025% (v/v) Triton X-100, 0.3M (w/v) glycine and 3% (w/v) BSA for 1h at room temperature. The section was then incubated with ab190565 (1/50) in TBS containing 0.025% (v/v) Triton X-100 and 3% (w/v) BSA overnight at +4°C. The section was then counterstained and mounted with SlowFade® Gold Antifade Mountant with DAPI.The DAPI only control (no antibody) inset shows no autoflourescence, demonstrating that any Alexa Fluor® 647 signal is dervied directly from bound ab190565. The separate images of ab190565 and DAPI alone, combined with the merged version of both signals, shows predominant co-localisation of the Alexa Fluor® 647 signal

IHC image of ab190565 staining in formalin fixed paraffin embedded tissue section of normal mouse cerebellum.The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH6) in a Dako Pascal pressure cooker using the standard factory-set regime. Non-specific protein-protein interactions were then blocked using in TBS containing 0.025% (v/v) Triton X-100, 0.3M (w/v) glycine and 3% (w/v) BSA for 1h at room temperature. The section was then incubated with ab190565 (1/50) in TBS containing 0.025% (v/v) Triton X-100 and 3% (w/v) BSA overnight at +4°C. The section was then counterstained and mounted with SlowFade® Gold Antifade Mountant with DAPI.The DAPI only control (no antibody) inset shows no autoflourescence, demonstrating that any Alexa Fluor® 647 signal is dervied directly from bound ab190565. The separate images of ab190565 and DAPI alone, combined with the merged version of both signals, shows predominant co-localisation of the Alexa Fluor® 647 sign
![ab190565 staining NeuN in NGF-differentiated PC12 cells (7 days). The cells were fixed with 100% methanol (5min) and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated with ab190565 at 1/50 dilution (shown in red) and ab7291 (Mouse monoclonal [DM1A] to alpha Tubulin) at 1µg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an Alexa Fluor® 488 Goat anti-Mouse secondary (ab150117) at 2 μg/ml (shown in green). Nuclear DNA was labelled in blue with DAPI.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/29212_ab190565-228185-ab190565AP200570410ugMeOHPC127d.jpg)
![ab190565 staining NeuN in U87-MG cells. The cells were fixed with 100% methanol (5min) and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated with ab190565 at 1/50 dilution(shown in red) and ab7291 (Mouse monoclonal [DM1A] to alpha Tubulin) at 1µg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an Alexa Fluor® 488 Goat anti-Mouse secondary (ab150117) at 2 μg/ml (shown in green). Nuclear DNA was labelled in blue with DAPI.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/29213_ab190565-230909-Layoutab190565directlyla.jpg)


