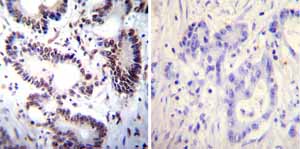
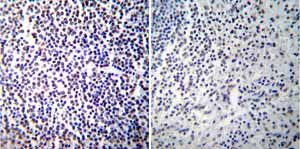
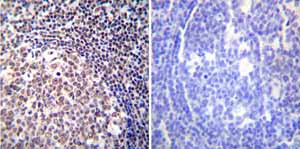
Anti-NFAT1 antibody [25A10.D6.D2] - ChIP Grade
| Name | Anti-NFAT1 antibody [25A10.D6.D2] - ChIP Grade |
|---|---|
| Supplier | Abcam |
| Catalog | ab2722 |
| Prices | $403.00 |
| Sizes | 100 µg |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG1 |
| Clone | 25A10.D6.D2 |
| Applications | IHC-P FC EMSA ICC/IF IP WB IHC-F ICC/IF ICC/IF ChIP |
| Species Reactivities | Mouse, Rat, Human |
| Antigen | Synthetic peptide corresponding to Mouse NFAT1 aa 51-69 |
| Description | Mouse Monoclonal |
| Gene | NFATC2 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Bookmarking promoters in mitotic chromatin: poly(ADP-ribose)polymerase-1 as an - Bookmarking promoters in mitotic chromatin: poly(ADP-ribose)polymerase-1 as an
Lodhi N, Kossenkov AV, Tulin AV. Nucleic Acids Res. 2014 Jun;42(11):7028-38.
NFAT1 and NFAT3 cooperate with HDAC4 during regulation of alternative splicing of - NFAT1 and NFAT3 cooperate with HDAC4 during regulation of alternative splicing of
Kosiorek M, Podszywalow-Bartnicka P, Zylinska L, Pikula S. PLoS One. 2014 Jun 6;9(6):e99118.
The ion channel TRPV1 regulates the activation and proinflammatory properties of - The ion channel TRPV1 regulates the activation and proinflammatory properties of
Bertin S, Aoki-Nonaka Y, de Jong PR, Nohara LL, Xu H, Stanwood SR, Srikanth S, Lee J, To K, Abramson L, Yu T, Han T, Touma R, Li X, Gonzalez-Navajas JM, Herdman S, Corr M, Fu G, Dong H, Gwack Y, Franco A, Jefferies WA, Raz E. Nat Immunol. 2014 Nov;15(11):1055-63.
Role of CTLA4 in the proliferation and survival of chronic lymphocytic leukemia. - Role of CTLA4 in the proliferation and survival of chronic lymphocytic leukemia.
Mittal AK, Chaturvedi NK, Rohlfsen RA, Gupta P, Joshi AD, Hegde GV, Bociek RG, Joshi SS. PLoS One. 2013 Aug 1;8(8):e70352.
CD3-T cell receptor co-stimulation through SLAMF3 and SLAMF6 receptors enhances - CD3-T cell receptor co-stimulation through SLAMF3 and SLAMF6 receptors enhances
Chatterjee M, Hedrich CM, Rauen T, Ioannidis C, Terhorst C, Tsokos GC. J Biol Chem. 2012 Nov 2;287(45):38168-77.
Minocycline suppresses activation of nuclear factor of activated T cells 1 - Minocycline suppresses activation of nuclear factor of activated T cells 1
Szeto GL, Pomerantz JL, Graham DR, Clements JE. J Biol Chem. 2011 Apr 1;286(13):11275-82.
TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental - TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental
Gonzalez-Navajas JM, Fine S, Law J, Datta SK, Nguyen KP, Yu M, Corr M, Katakura K, Eckman L, Lee J, Raz E. J Clin Invest. 2010 Feb;120(2):570-81.
NIP45 controls the magnitude of the type 2 T helper cell response. - NIP45 controls the magnitude of the type 2 T helper cell response.
Fathman JW, Gurish MF, Hemmers S, Bonham K, Friend DS, Grusby MJ, Glimcher LH, Mowen KA. Proc Natl Acad Sci U S A. 2010 Feb 23;107(8):3663-8. doi:
NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed - NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed
Dammermann W, Zhang B, Nebel M, Cordiglieri C, Odoardi F, Kirchberger T, Kawakami N, Dowden J, Schmid F, Dornmair K, Hohenegger M, Flugel A, Guse AH, Potter BV. Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10678-83. doi:
Cognitive decline in Alzheimer's disease is associated with selective changes in - Cognitive decline in Alzheimer's disease is associated with selective changes in
Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H 3rd, Kraner SD, Norris CM. J Neurosci. 2009 Oct 14;29(41):12957-69.
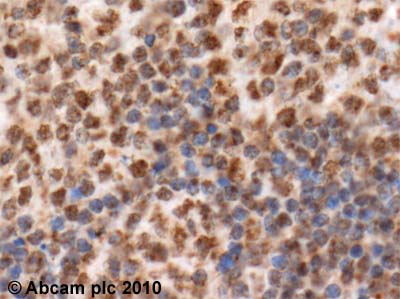
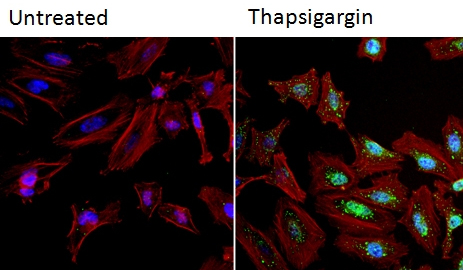
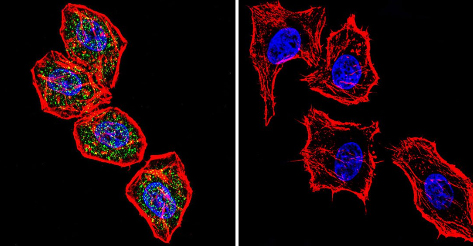
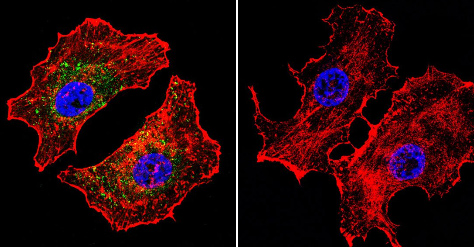
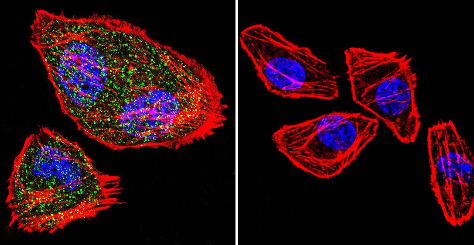
![Anti-NFAT1 antibody [25A10.D6.D2] - ChIP Grade (ab2722) at 1 µg/ml + Spleen (Human) Tissue Lysate - adult normal tissue (ab29699) at 10 µgSecondaryGoat Anti-Mouse IgG H&L (HRP) preadsorbed (ab97040) at 1/5000 dilution ( )developed using the ECL techniquePerformed under reducing conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/29828_NFAT1-Primary-antibodies-ab2722-3.jpg)
![Overlay histogram showing Jurkat cells stained with ab2722 (red line). The cells were fixed with 80% methanol (5 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab2722, 2µg/1x106 cells) for 30 min at 22ºC. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_3/29829_NFAT1-Primary-antibodies-ab2722-4.jpg)