
Breast carcinoma tissue was fixed in 10% formalin at 4°C overnight and paraffin embedded. Following blocking the tissue sections were incubated with ab4514 (1/50 dilution) at 37°C for 1 hour, and with the secondary antibody at 37°C for 30 minutes. The slides were counterstained with Hematoxylin. Washes were carried out using TBS. Heat mediated antigen retrieval was carried out using the Microwave method before commencing the staining protocol. ab4514 staining appears to be localized mainly to the cytoplasm. Although the putative cellular localization of NSD3 is the nucleus we have found no evidence in the current literature to confirm this and we believe ab4514 may be detecting a cytoplasmic form of the protein. We would be very grateful for any customer feedback about NSD3 and ab4514.

All lanes : Anti-NSD3 antibody (ab4514) at 1 µg/mlLane 1 : HeLa whole cell extractLane 2 : HEK293 cell extractLane 3 : HeLa whole cell extract with Human NSD3 peptide (ab14995) at 1 µg/mlLane 4 : HEK293 cell extract with Human NSD3 peptide (ab14995) at 1 µg/mlLysates/proteins at 20 µg per lane.
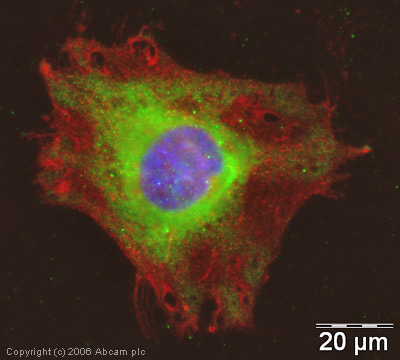
ICC/IF image of ab4514 stained human HeLa cells. The cells were methanol fixed (5 min) and incubated with the antibody (ab4514, 5µg/ml) for 1h at room temperature. The secondary antibody (green) was Alexa Fluor® 488 goat anti-rabbit IgG (H+L) used at a 1/1000 dilution for 1h. Image-iTTM FX Signal Enhancer was used as the primary blocking agent, 5% BSA (in TBS-T) was used for all other blocking steps. DAPI was used to stain the cell nuclei (blue). Alexa Fluor® 594 WGA was used to label plasma membranes (red). As seen in Immunohistochemical analysis (shown above), although the putative cellular localization of NSD3 is the nucleus we have found no evidence in the current literature to confirm this and we believe ab4514 may be detecting a cytoplasmic form of the protein.


