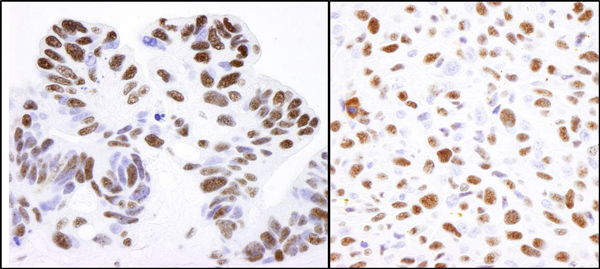
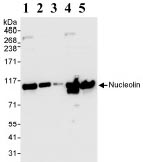
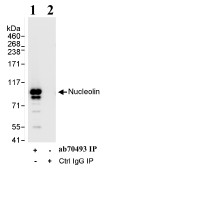
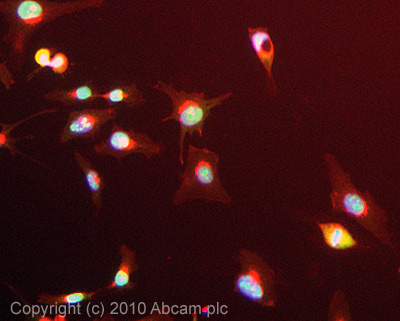
Anti-Nucleolin antibody
| Name | Anti-Nucleolin antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab70493 |
| Prices | $398.00 |
| Sizes | 100 µl |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | IHC-P ICC/IF ICC/IF WB IP |
| Species Reactivities | Mouse, Human, Rabbit, Horse, Guinea Pig, Bovine, Dog, Pig, Chimpanzee, Monkey, Ape, Monkey, Orangutan, Elephant |
| Antigen | Synthetic peptide corresponding to a region between residue 550 and the C-terminus (residue 710) of human Nucleolin |
| Description | Rabbit Polyclonal |
| Gene | NCL |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
The maternal nucleolus plays a key role in centromere satellite maintenance - The maternal nucleolus plays a key role in centromere satellite maintenance
Fulka H, Langerova A. Development. 2014 Apr;141(8):1694-704.
ANKRD1 acts as a transcriptional repressor of MMP13 via the AP-1 site. - ANKRD1 acts as a transcriptional repressor of MMP13 via the AP-1 site.
Almodovar-Garcia K, Kwon M, Samaras SE, Davidson JM. Mol Cell Biol. 2014 Apr;34(8):1500-11.
Nucleolin mediates nucleosome disruption critical for DNA double-strand break - Nucleolin mediates nucleosome disruption critical for DNA double-strand break
Goldstein M, Derheimer FA, Tait-Mulder J, Kastan MB. Proc Natl Acad Sci U S A. 2013 Oct 15;110(42):16874-9. doi: