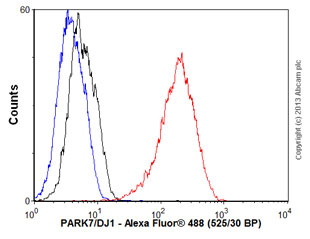
Overlay histogram showing HepG2 cells stained with ab76008 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab76008, 1/100 dilution) for 30 min at 22°C. The secondary antibody used was Alexa Fluor® 488 goat anti-rabbit IgG (H+L) (ab150077) at 1/2000 dilution for 30 min at 22°C. Isotype control antibody (black line) was rabbit IgG (monoclonal) (1μg/1x106 cells) used under the same conditions. Unlabelled sample (blue line) was also used as a control. Acquisition of >5,000 events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter. This antibody gave a positive signal in HepG2 cells fixed with 80% methanol (5 min)/permeabilized with 0.1% PBS-Tween for 20 min used under the same conditions.
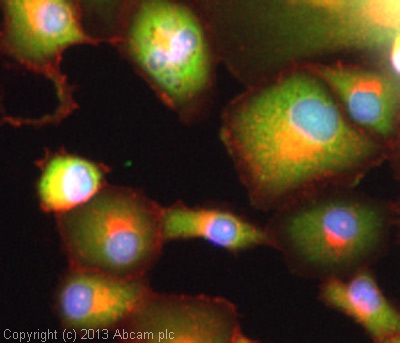
ICC/IF image of ab76008 stained PANC-1 cells. The cells were 4% formaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab76008, 1/50 dilution) overnight at +4°C. The secondary antibody (green) was ab96899, DyLight® 488 goat anti-rabbit IgG (H+L) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.
![All lanes : Anti-PARK7/DJ1 antibody [EP2815Y] (ab76008) at 1/20000 dilutionLane 1 : Jurkat cell lysateLane 2 : HeLa cell lysateLane 3 : NIH3T3 cell lysateLane 4 : 293T cell lysateLysates/proteins at 10 µg per lane.SecondaryGoat anti-rabbit HRP at 1/1000 dilution](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_4/7308_ab76008-1.jpg)
All lanes : Anti-PARK7/DJ1 antibody [EP2815Y] (ab76008) at 1/20000 dilutionLane 1 : Jurkat cell lysateLane 2 : HeLa cell lysateLane 3 : NIH3T3 cell lysateLane 4 : 293T cell lysateLysates/proteins at 10 µg per lane.SecondaryGoat anti-rabbit HRP at 1/1000 dilution
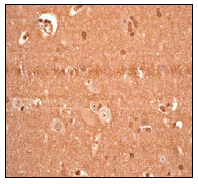
ab76008, at 1/250 dilution, staining PARK7/DJ1 in human brain by immunohistochemistry using paraffin-embedded tissue.


![All lanes : Anti-PARK7/DJ1 antibody [EP2815Y] (ab76008) at 1/20000 dilutionLane 1 : Jurkat cell lysateLane 2 : HeLa cell lysateLane 3 : NIH3T3 cell lysateLane 4 : 293T cell lysateLysates/proteins at 10 µg per lane.SecondaryGoat anti-rabbit HRP at 1/1000 dilution](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_4/7308_ab76008-1.jpg)
