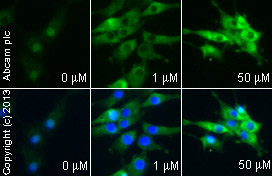
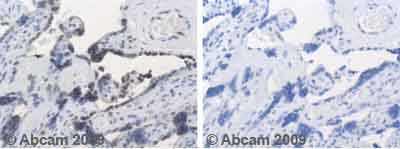
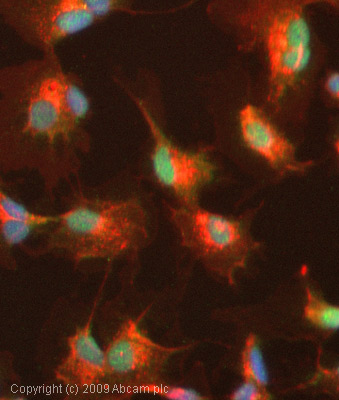
Anti-PPAR gamma antibody
| Name | Anti-PPAR gamma antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab19481 |
| Prices | $390.00 |
| Sizes | 200 µg |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | ICC/IF ICC/IF IHC-P WB |
| Species Reactivities | Mouse, Rat, Chicken, Hamster, Bovine, Dog, Human, Pig |
| Antigen | Synthetic peptide (Human) (C terminal) from a region between AA 420 and 460 of the PPARgamma protein |
| Description | Rabbit Polyclonal |
| Gene | PPARG |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Combined blockade of angiotensin II type 1 receptor and activation of peroxisome - Combined blockade of angiotensin II type 1 receptor and activation of peroxisome
Nenicu A, Korbel C, Gu Y, Menger MD, Laschke MW. Hum Reprod. 2014 May;29(5):1011-24.
A network pharmacology study of Chinese medicine QiShenYiQi to reveal its - A network pharmacology study of Chinese medicine QiShenYiQi to reveal its
Li X, Wu L, Liu W, Jin Y, Chen Q, Wang L, Fan X, Li Z, Cheng Y. PLoS One. 2014 May 9;9(5):e95004.
Altered peroxisome-proliferator activated receptors expression in human - Altered peroxisome-proliferator activated receptors expression in human
Knapp P, Chabowski A, Blachnio-Zabielska A, Jarzabek K, Wolczynski S. PPAR Res. 2012;2012:471524.
ERK1 and ERK2 are involved in recruitment and maturation of human mesenchymal - ERK1 and ERK2 are involved in recruitment and maturation of human mesenchymal
Donzelli E, Lucchini C, Ballarini E, Scuteri A, Carini F, Tredici G, Miloso M. J Mol Cell Biol. 2011 Apr;3(2):123-31.
Impaired apoptotic cell clearance in CGD due to altered macrophage programming is - Impaired apoptotic cell clearance in CGD due to altered macrophage programming is
Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, Henson PM, Bratton DL. Blood. 2009 Feb 26;113(9):2047-55.
Expression of peroxisome proliferator-activated receptor-gamma in key neuronal - Expression of peroxisome proliferator-activated receptor-gamma in key neuronal
Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, Schwartz MW. Endocrinology. 2009 Feb;150(2):707-12.
Angiotensin II induces inflammatory response partly via toll-like receptor - Angiotensin II induces inflammatory response partly via toll-like receptor
Ji Y, Liu J, Wang Z, Liu N. Cell Physiol Biochem. 2009;23(4-6):265-76.
Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by - Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by
Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Mol Pharmacol. 2008 Feb;73(2):399-409. Epub 2007 Nov 15.