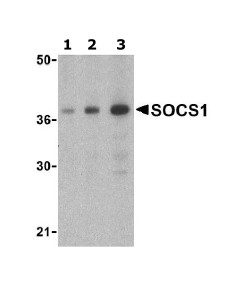
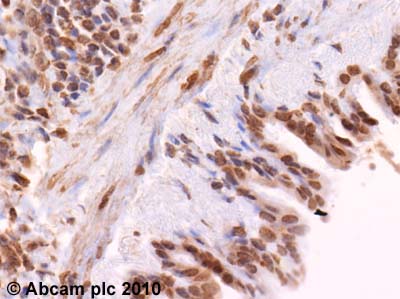
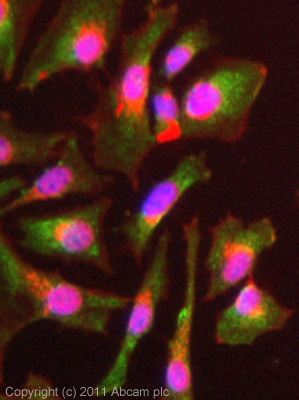
Anti-SOCS1 antibody
| Name | Anti-SOCS1 antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab62584 |
| Prices | $385.00 |
| Sizes | 100 µg |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | WB ICC/IF ICC/IF IHC-P |
| Species Reactivities | Mouse, Rat, Human |
| Antigen | Synthetic 15 amino acid peptide from near the carboxy terminus of human SOCS1 |
| Description | Rabbit Polyclonal |
| Gene | SOCS1 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Interleukin-1beta promotes ovarian tumorigenesis through a p53/NF-kappaB-mediated - Interleukin-1beta promotes ovarian tumorigenesis through a p53/NF-kappaB-mediated
Schauer IG, Zhang J, Xing Z, Guo X, Mercado-Uribe I, Sood AK, Huang P, Liu J. Neoplasia. 2013 Apr;15(4):409-20.
Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by - Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by
Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Immunity. 2009 Jan 16;30(1):80-91.