![Immuofluorescent staining for SynGap phospho S1123 in the hippocampus of a naïve rat using antibody ab16649. [A] Immunostaining was observed in the cytoplasm of a few hippocampal neurons (Picture taken with a X10 objective). Higher power magnification of the same hippocampal section is shown in image B (X 40 objective). Tissue preparation: rat brain tissue was perfusion fixed (4% PFA) followed by post fix and cryoprotection in 20% sucrose before freezing in OCT. 30µm coronal sections were cut on a cryostat for free floating IHC. Primary antibody ab16649 was used at 1/3000 (0.2µg/ml) incubated overnight at room temperature. Secondary antibody used: anti-rabbit Alexa fluor 488 (1/1000) incubated for 2 hours at room temperature.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_5/7611_ab16649_2.jpg)
Immuofluorescent staining for SynGap phospho S1123 in the hippocampus of a naïve rat using antibody ab16649. [A] Immunostaining was observed in the cytoplasm of a few hippocampal neurons (Picture taken with a X10 objective). Higher power magnification of the same hippocampal section is shown in image B (X 40 objective). Tissue preparation: rat brain tissue was perfusion fixed (4% PFA) followed by post fix and cryoprotection in 20% sucrose before freezing in OCT. 30µm coronal sections were cut on a cryostat for free floating IHC. Primary antibody ab16649 was used at 1/3000 (0.2µg/ml) incubated overnight at room temperature. Secondary antibody used: anti-rabbit Alexa fluor 488 (1/1000) incubated for 2 hours at room temperature.
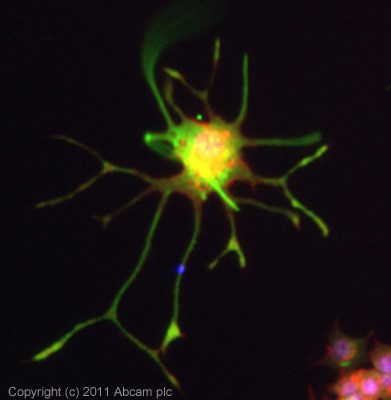
ICC/IF image of ab16649 stained PC12 cells. The cells were 4% PFA fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab16649, 1µg/ml) overnight at +4°C. The secondary antibody (green) was ab96899 Dylight 488 goat anti-rabbit IgG (H+L) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.
![Immuofluorescent staining for SynGap phospho S1123 in the hippocampus of a naïve rat using antibody ab16649. [A] Immunostaining was observed in the cytoplasm of a few hippocampal neurons (Picture taken with a X10 objective). Higher power magnification of the same hippocampal section is shown in image B (X 40 objective). Tissue preparation: rat brain tissue was perfusion fixed (4% PFA) followed by post fix and cryoprotection in 20% sucrose before freezing in OCT. 30µm coronal sections were cut on a cryostat for free floating IHC. Primary antibody ab16649 was used at 1/3000 (0.2µg/ml) incubated overnight at room temperature. Secondary antibody used: anti-rabbit Alexa fluor 488 (1/1000) incubated for 2 hours at room temperature.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_5/7611_ab16649_2.jpg)
