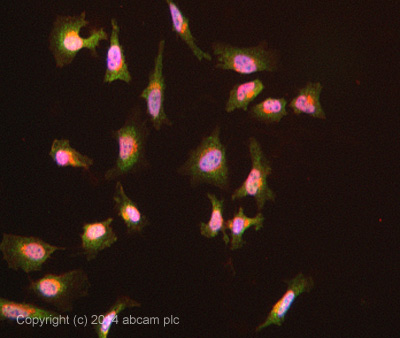
ab108216 stained HeLa cells. The cells were 4% formaldehyde fixed for 10 minutes at room temperature and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1hour at room temperature to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab108216 at 5µg/ml) overnight at +4°C. The secondary antibody (pseudo-colored green) was Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) preadsorbed (ab150081) used at a 1/1000 dilution for 1hour at room temperature. Alexa Fluor® 594 WGA was used to label plasma membranes (pseudo-colored red) at a 1/200 dilution for 1hour at room temperature. DAPI was used to stain the cell nuclei (pseudo-colored blue) at a concentration of 1.43µM for 1hour at room temperature.
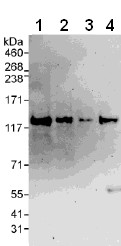
All lanes : Anti-VARP antibody (ab108216) at 0.1 µg/mlLane 1 : HeLa whole cell lysate at 50 µgLane 2 : HeLa whole cell lysate at 15 µgLane 3 : HeLa whole cell lysate at 5 µgLane 4 : 293T whole cell lysate at 50 µgdeveloped using the ECL technique
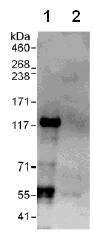
Detection of VARP by Western Blot of Immunprecipitate. Lane 1: ab108216 at 1µg/ml staining VARP in HeLa whole cell lysate immunoprecipitated using ab108216 at 6µg/mg lysate (1 mg/IP; 20% of IP loaded/lane). Lane 2: Control IgG Detection: Chemiluminescence with exposure time of 30 seconds.


