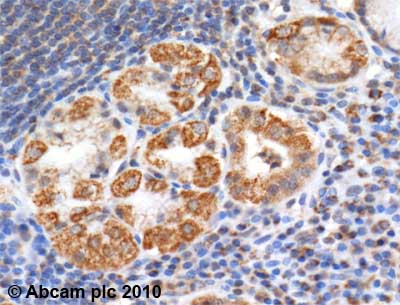
Anti-Aconitase 2 antibody
| Name | Anti-Aconitase 2 antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab71440 |
| Prices | $398.00 |
| Sizes | 400 µl |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | IHC-P WB |
| Species Reactivities | Mouse, Rat, Human |
| Antigen | Synthetic peptide (Human) conjugated to KLH |
| Description | Rabbit Polyclonal |
| Gene | ACO2 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial - Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial
Kim SJ, Cheresh P, Williams D, Cheng Y, Ridge K, Schumacker PT, Weitzman S, Bohr VA, Kamp DW. J Biol Chem. 2014 Feb 28;289(9):6165-76.
Functional proteomic analysis reveals sex-dependent differences in structural and - Functional proteomic analysis reveals sex-dependent differences in structural and
Fogle RL, Hollenbeak CS, Stanley BA, Vary TC, Kimball SR, Lynch CJ. Physiol Genomics. 2011 Apr 12;43(7):346-56. doi:
Identification of a redox-modulatory interaction between uncoupling protein 3 and - Identification of a redox-modulatory interaction between uncoupling protein 3 and
Hirasaka K, Lago CU, Kenaston MA, Fathe K, Nowinski SM, Nikawa T, Mills EM. Antioxid Redox Signal. 2011 Nov 15;15(10):2645-61.

![All lanes : Anti-Aconitase 2 antibody (ab71440) at 1/1000 dilutionLane 1 : Perfused isolated rat heart whole tissue lysate lysed with 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS, 0.5% Na-deoxycholate, 1 mM Na3VO4, 20 mM NaF, 1 mM PMSF, 5 v/v % protease inhibitor cocktail Lane 2 : Perfused isolated rat heart whole tissue lysate lysed with T-PER Tissue Protein Extraction Reagent [# 785101; Pierce], containing 1mM Na3VO4, 20 mM NaF, 5 v/v % protease inhibitor cocktail Lysates/proteins at 12.5 µg per lane.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/1870_actn2.jpg)