![Annexin A1 was immunoprecipitated using 0.5mg Hela whole cell extract, 5µg of Rabbit polyclonal to Annexin A1 and 50µl of protein G magnetic beads (+). No antibody was added to the control (-).The antibody was incubated under agitation with Protein G beads for 10min, Hela whole cell extract lysate diluted in RIPA buffer was added to each sample and incubated for a further 10min under agitation.Proteins were eluted by addition of 40µl SDS loading buffer and incubated for 10min at 70C; 10µl of each sample was separated on a SDS PAGE gel, transferred to a nitrocellulose membrane, blocked with 5% BSA and probed with ab65844.Secondary: Mouse monoclonal [SB62a] Secondary Antibody to Rabbit IgG light chain (HRP) (ab99697).Band: 39kDa; Annexin A1, non specific bands - 52kDa and in control (lane 2): We are unsure as to the identity of this extra band.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/7428_ab65844-199604-IPV024ab658448m.jpg)
Annexin A1 was immunoprecipitated using 0.5mg Hela whole cell extract, 5µg of Rabbit polyclonal to Annexin A1 and 50µl of protein G magnetic beads (+). No antibody was added to the control (-).The antibody was incubated under agitation with Protein G beads for 10min, Hela whole cell extract lysate diluted in RIPA buffer was added to each sample and incubated for a further 10min under agitation.Proteins were eluted by addition of 40µl SDS loading buffer and incubated for 10min at 70C; 10µl of each sample was separated on a SDS PAGE gel, transferred to a nitrocellulose membrane, blocked with 5% BSA and probed with ab65844.Secondary: Mouse monoclonal [SB62a] Secondary Antibody to Rabbit IgG light chain (HRP) (ab99697).Band: 39kDa; Annexin A1, non specific bands - 52kDa and in control (lane 2): We are unsure as to the identity of this extra band.

ab65844 staining Annexin A1 in human mammary cancer tissue by IHC-P (Formaldehyde/PFA-fixed, paraffin-embedded immunohistochemistry).
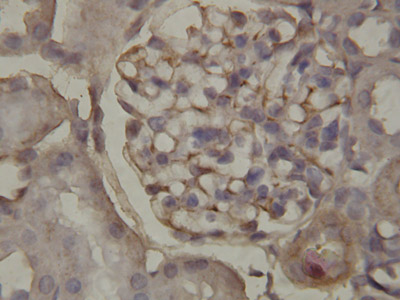
ab65844 staining Annexin A1 in rat kidney tissue sections by IHC-P (Formaldehyde/PFA-fixed, paraffin-embedded immunohistochemistry).
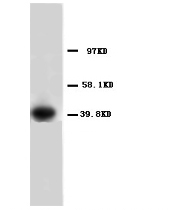
Anti-Annexin A1 antibody - Carboxyterminal end (ab65844) + HeLa cell lysate
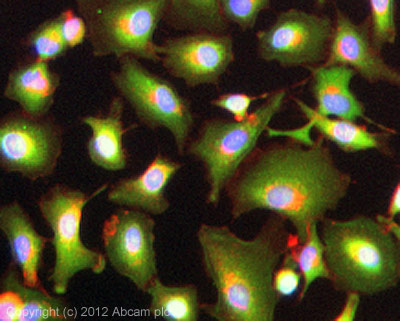
ICC/IF image of ab65844 stained A549 cells. The cells were 100% methanol fixed (5 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab65844, 5µg/ml) overnight at +4°C. The secondary antibody (green) was ab96899, DyLight® 488 goat anti-rabbit IgG (H+L) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.
![Annexin A1 was immunoprecipitated using 0.5mg Hela whole cell extract, 5µg of Rabbit polyclonal to Annexin A1 and 50µl of protein G magnetic beads (+). No antibody was added to the control (-).The antibody was incubated under agitation with Protein G beads for 10min, Hela whole cell extract lysate diluted in RIPA buffer was added to each sample and incubated for a further 10min under agitation.Proteins were eluted by addition of 40µl SDS loading buffer and incubated for 10min at 70C; 10µl of each sample was separated on a SDS PAGE gel, transferred to a nitrocellulose membrane, blocked with 5% BSA and probed with ab65844.Secondary: Mouse monoclonal [SB62a] Secondary Antibody to Rabbit IgG light chain (HRP) (ab99697).Band: 39kDa; Annexin A1, non specific bands - 52kDa and in control (lane 2): We are unsure as to the identity of this extra band.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/7428_ab65844-199604-IPV024ab658448m.jpg)
