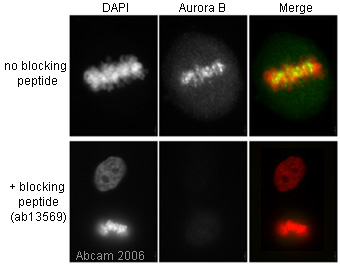
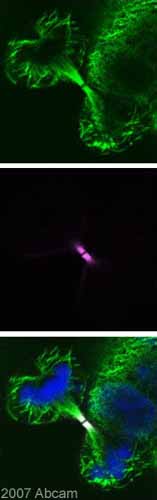
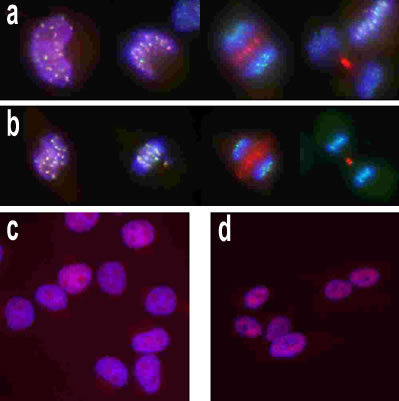
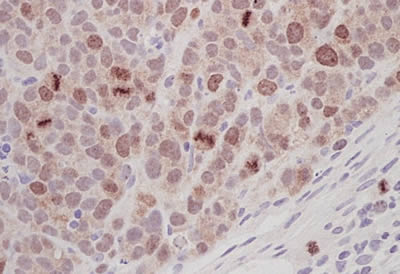
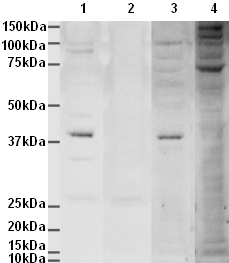
Anti-Aurora B antibody
| Name | Anti-Aurora B antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab2254 |
| Prices | $400.00 |
| Sizes | 100 µg |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | ICC/IF ICC/IF IHC-F IHC-P IP WB |
| Species Reactivities | Mouse, Rat, Hamster, Human, Pig |
| Antigen | Synthetic peptide conjugated to KLH derived from within residues 1 - 100 of Human Aurora B |
| Description | Rabbit Polyclonal |
| Gene | AURKB |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
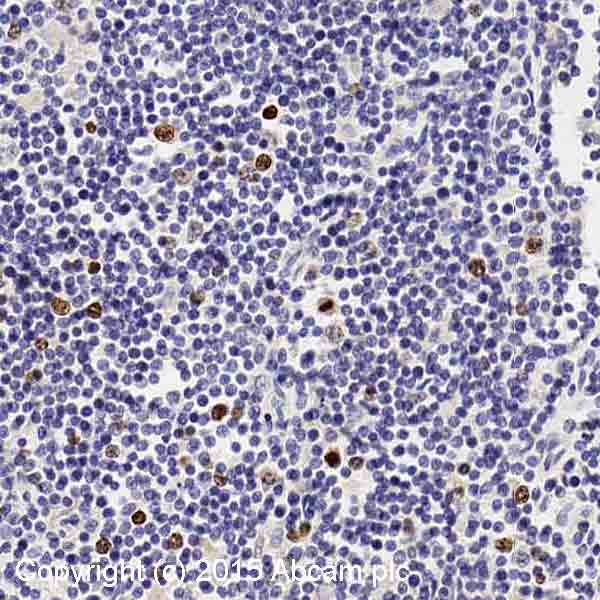
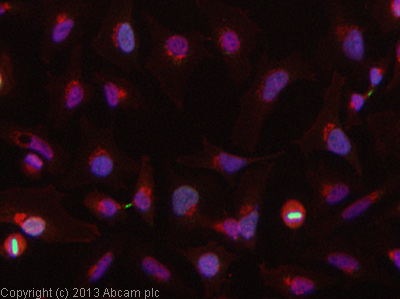
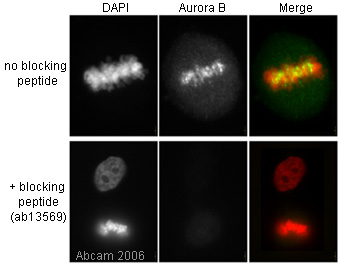
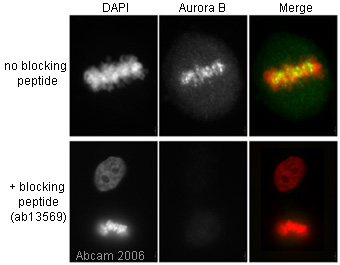
Product images
Product References
Selective disruption of aurora C kinase reveals distinct functions from aurora B - Selective disruption of aurora C kinase reveals distinct functions from aurora B
Balboula AZ, Schindler K. PLoS Genet. 2014 Feb 27;10(2):e1004194.
EB1 enables spindle microtubules to regulate centromeric recruitment of Aurora B. - EB1 enables spindle microtubules to regulate centromeric recruitment of Aurora B.
Banerjee B, Kestner CA, Stukenberg PT. J Cell Biol. 2014 Mar 17;204(6):947-63.
Chk2 prevents mitotic exit when the majority of kinetochores are unattached. - Chk2 prevents mitotic exit when the majority of kinetochores are unattached.
Petsalaki E, Zachos G. J Cell Biol. 2014 May 12;205(3):339-56.
Alisertib added to rituximab and vincristine is synthetic lethal and potentially - Alisertib added to rituximab and vincristine is synthetic lethal and potentially
Mahadevan D, Morales C, Cooke LS, Manziello A, Mount DW, Persky DO, Fisher RI, Miller TP, Qi W. PLoS One. 2014 Jun 3;9(6):e95184.
TAO1 kinase maintains chromosomal stability by facilitating proper congression of - TAO1 kinase maintains chromosomal stability by facilitating proper congression of
Shrestha RL, Tamura N, Fries A, Levin N, Clark J, Draviam VM. Open Biol. 2014 Jun;4(6):130108.
WEE1 murine deficiency induces hyper-activation of APC/C and results in genomic - WEE1 murine deficiency induces hyper-activation of APC/C and results in genomic
Vassilopoulos A, Tominaga Y, Kim HS, Lahusen T, Li B, Yu H, Gius D, Deng CX. Oncogene. 2014 Aug 4;0.
Aurora B prevents delayed DNA replication and premature mitotic exit by - Aurora B prevents delayed DNA replication and premature mitotic exit by
Trakala M, Fernandez-Miranda G, Perez de Castro I, Heeschen C, Malumbres M. Cell Cycle. 2013 Apr 1;12(7):1030-41.
Novel pyrimidine-2,4-diamine derivative suppresses the cell viability and spindle - Novel pyrimidine-2,4-diamine derivative suppresses the cell viability and spindle
Salmela AL, Pouwels J, Maki-Jouppila J, Kohonen P, Toivonen P, Kallio L, Kallio M. Carcinogenesis. 2013 Feb;34(2):436-45.
Stimulating myocardial regeneration with periostin Peptide in large mammals - Stimulating myocardial regeneration with periostin Peptide in large mammals
Ladage D, Yaniz-Galende E, Rapti K, Ishikawa K, Tilemann L, Shapiro S, Takewa Y, Muller-Ehmsen J, Schwarz M, Garcia MJ, Sanz J, Hajjar RJ, Kawase Y. PLoS One. 2013 May 20;8(5):e59656.
The Prp19 complex directly functions in mitotic spindle assembly. - The Prp19 complex directly functions in mitotic spindle assembly.
Hofmann JC, Tegha-Dunghu J, Drager S, Will CL, Luhrmann R, Gruss OJ. PLoS One. 2013 Sep 19;8(9):e74851.