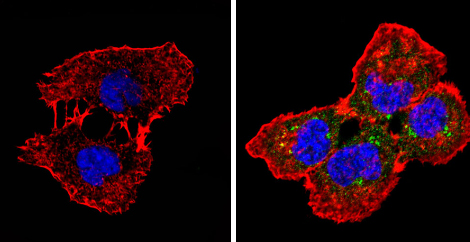
Immunocytochemistry/Immunofluorescence analysis of beta 1 Spectrin (green) showing staining in the cytoplasm of A431 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were incubated with ab2808 in 3% BSA-PBS at a dilution of 1:20 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
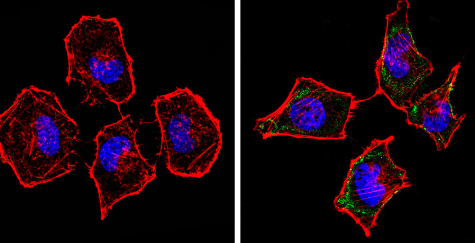
Immunocytochemistry/Immunofluorescence analysis of beta 1 Spectrin (green) showing staining in the cytoplasm of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were incubated with ab2808 in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
![Anti-beta 1 Spectrin antibody [4C3] (ab2808) at 1/1000 dilution + HeLa cell lysate at 25 µg](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/13490_ab2808-219067-ab2808wb.jpg)
Anti-beta 1 Spectrin antibody [4C3] (ab2808) at 1/1000 dilution + HeLa cell lysate at 25 µg
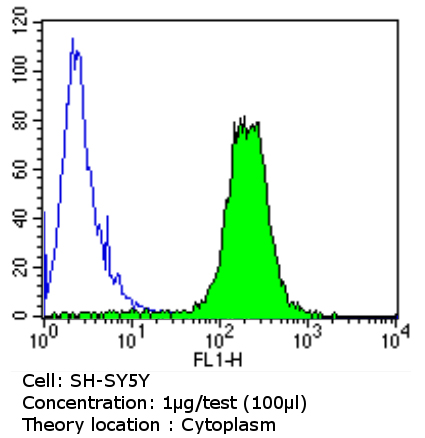
Flow cytometry analysis of beta 1 Spectrin showing positive staining in the cytoplasm of SH-SY5Y cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/ml, fixed with 2% paraformaldehyde and washed with PBS. Cells were penetrated by dropping the supernatant, adding 90% methanol and incubated for 10 minutes at room temperature. Follwing penetration, cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with ab2808 (1 ug/test) for 60 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
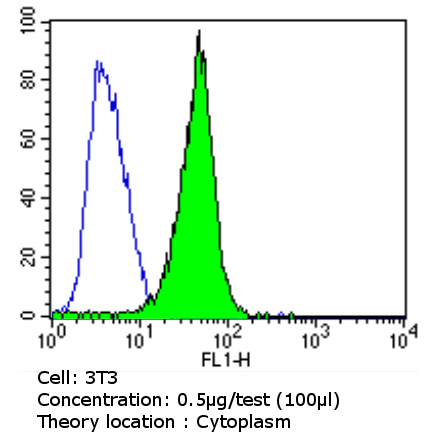
Flow cytometry analysis of beta 1 Spectrin showing positive staining in the cytoplasm of NIH/3T3 cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/ml, fixed with 2% paraformaldehyde and washed with PBS. Cells were penetrated by dropping the supernatant, adding 90% methanol and incubated for 10 minutes at room temperature. Follwing penetration, cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with ab2808 (0.5 ug/test) for 60 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
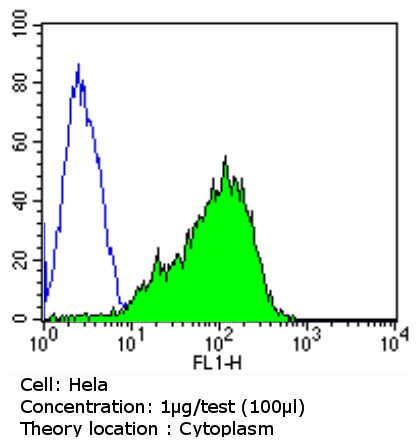
Flow cytometry analysis of beta 1 Spectrin showing positive staining in the cytoplasm of Hela cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/ml, fixed with 2% paraformaldehyde and washed with PBS. Cells were penetrated by dropping the supernatant, adding 90% methanol and incubated for 10 minutes at room temperature. Follwing penetration, cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with ab2808 (1 ug/test) for 60 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
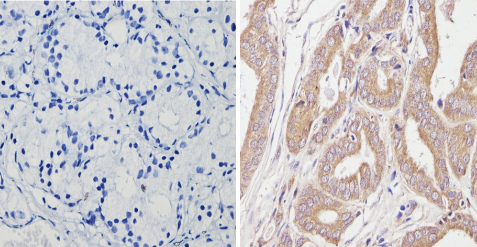
ab2808 labelling beta 1 Spectrin in the cytoplasm of Human prostate carcinoma (right) compared with a negative control (left) by Immunohistochemistry (formalin-PFA-fixed paraffin-embedded sections). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature. Tissue sections were incubated with the primary antibody (1:20 in 3% BSA-PBS) overnight at 4°C. A HRP-conjugated anti-mouse was used as the secondary antibody, followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
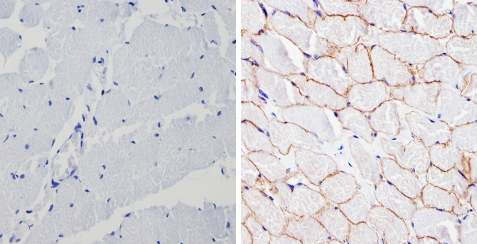
ab2808 labelling beta 1 Spectrin in the membrane of Human skeletal muscle (right) compared with a negative control (left) by Immunohistochemistry (formalin-PFA-fixed paraffin-embedded sections). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature. Tissue sections were incubated with the primary antibody (1:200 in 3% BSA-PBS) overnight at 4°C. A HRP-conjugated anti-mouse was used as the secondary antibody, followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
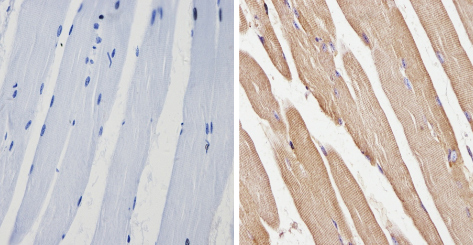
ab2808 labelling beta 1 Spectrin in the cytoplasm and membrane of Mouse skeletal muscle (right) compared with a negative control (left) by Immunohistochemistry (formalin-PFA-fixed paraffin-embedded sections). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature. Tissue sections were incubated with the primary antibody (1:20 in 3% BSA-PBS) overnight at 4°C. A HRP-conjugated anti-mouse was used as the secondary antibody, followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.

IHC image of ab2808 staining in human skeletal muscle formalin fixed paraffin embedded tissue section, performed on a Leica BondTM system using the standard protocol F. The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH6, epitope retrieval solution 1) for 20 mins. The section was then incubated with ab2808, 5µg/ml, for 15 mins at room temperature and detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The section was then counterstained with haematoxylin and mounted with DPX.For other IHC staining systems (automated and non-automated) customers should optimize variable parameters such as antigen retrieval conditions, primary antibody concentration and antibody incubation times.
![Overlay histogram showing SH-SY5Y cells stained with ab2808 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab2808, 2µg/1x106 cells dilution) for 30 min at 22ºC. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/13498_beta-1-Spectrin-Primary-antibodies-ab2808-2.jpg)
Overlay histogram showing SH-SY5Y cells stained with ab2808 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab2808, 2µg/1x106 cells dilution) for 30 min at 22ºC. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed.


![Anti-beta 1 Spectrin antibody [4C3] (ab2808) at 1/1000 dilution + HeLa cell lysate at 25 µg](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/13490_ab2808-219067-ab2808wb.jpg)







![Overlay histogram showing SH-SY5Y cells stained with ab2808 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab2808, 2µg/1x106 cells dilution) for 30 min at 22ºC. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/13498_beta-1-Spectrin-Primary-antibodies-ab2808-2.jpg)