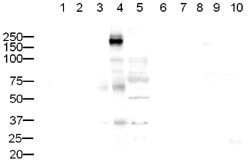
ab10559 rabbit polyclonal to CD45 (1/500) with secondary goat anti-rabbit IgG ab6721 (1/5000). Expected molecular weight: 147 kDa. WB exposure time: 1min 30sec Lanes 1 to 10: 20µg of cell lysate per laneLanes 6 to 10: ab17550 CD45 blocking peptide used at 1µg/ml Lane 1: HeLa Nuclear Extract (ab10559)Lane 2: HeLa Whole Cell Lysate (ab10559)Lane 3: A431 Whole Cell Lysate (ab10559)Lane 4: Jurkat Whole Cell Lysate (ab10559)Lane 5: HEK293 Whole Cell Lysate (ab10559)Lane 6: HeLa Nuclear Extract (ab10559+ ab17550)Lane 7: HeLa Whole Cell Lysate (ab10559+ ab17550)Lane 8: A431 Whole Cell Lysate (ab10559+ ab17550)Lane 9: Jurkat Whole Cell Lysate (ab10559+ ab17550)Lane 10: HEK293 Whole Cell Lysate (ab10559+ ab17550)A strong band, slightly higher was seen in Jurkat cell lysate. The band was blocked using the immunising peptide (ab17550). It is likely that the band is CD45.

Asynchronous KM-H2 cells were pelleted and labeled by indirect immunofluorescence. Cells were stained with ab10559 (1/200) for 30min at 4'C, washed and then stained with goat anti-rabbit alexafluor 488 (1/200). Forward/Side scatter were used to eliminate cellular debris. The accompanying marker was applied such that only 2% of the IgG control was positive. Based on the accompanying image, approximately 9.62% of cells exhibited positive staining for anti-CD45. Since KM-H2 have low levels of CD45 transcripts, it is expected that they have low levels of CD45 on their surface which is reflected in the ~9% positive. This image is taken from an Abreview.
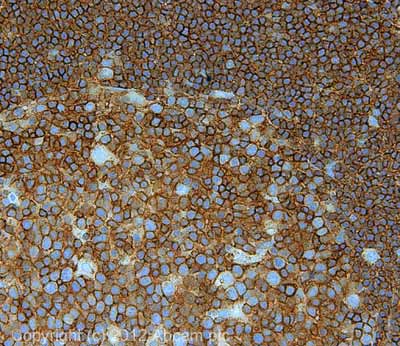
IHC image of ab10559 staining in human Hodgkins lymphoma formalin fixed paraffin embedded tissue section, performed on a Leica BondTM system using the standard protocol F. The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH6, epitope retrieval solution 1) for 20 mins. The section was then incubated with ab10559, 5µg/ml, for 15 mins at room temperature and detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The section was then counterstained with haematoxylin and mounted with DPX. For other IHC staining systems (automated and non-automated) customers should optimize variable parameters such as antigen retrieval conditions, primary antibody concentration and antibody incubation times.


