Anti-Cyclophilin A antibody
| Name | Anti-Cyclophilin A antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab58144 |
| Prices | $403.00 |
| Sizes | 100 µg |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG2a |
| Applications | WB ICC/IF ICC/IF ELISA IP FC |
| Species Reactivities | Human |
| Antigen | Recombinant full length protein, corresponding to amino acids 1-166 of Human Cyclophilin A |
| Description | Mouse Monoclonal |
| Gene | PPIA |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Proteomic profiling of high glucose primed monocytes identifies cyclophilin A as - Proteomic profiling of high glucose primed monocytes identifies cyclophilin A as
Ramachandran S, Venugopal A, Sathisha K, Reshmi G, Charles S, Divya G, Chandran NS, Mullassari A, Pillai MR, Kartha CC. Proteomics. 2012 Sep;12(18):2808-21.
Decreased levels of AKR1B1 and AKR1B10 in cancerous endometrium compared to - Decreased levels of AKR1B1 and AKR1B10 in cancerous endometrium compared to
Hevir N, Sinkovec J, Lanisnik Rizner T. Chem Biol Interact. 2013 Feb 25;202(1-3):226-33.
Disturbed expression of phase I and phase II estrogen-metabolizing enzymes in - Disturbed expression of phase I and phase II estrogen-metabolizing enzymes in
Hevir N, Sinkovec J, Rizner TL. Mol Cell Endocrinol. 2011 Jan 1;331(1):158-67.
Cyclosporine A inhibits hepatitis C virus nonstructural protein 2 through - Cyclosporine A inhibits hepatitis C virus nonstructural protein 2 through
Ciesek S, Steinmann E, Wedemeyer H, Manns MP, Neyts J, Tautz N, Madan V, Bartenschlager R, von Hahn T, Pietschmann T. Hepatology. 2009 Nov;50(5):1638-45.
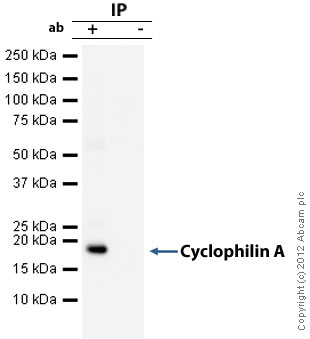

![Overlay histogram showing HeLa cells stained with ab58144 (red line). The cells were fixed with 4% paraformaldehyde and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab58144, 0.5µg/1x106 cells) for 30 min at 22ºC. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG2a [ICIGG2A] (ab91361, 1µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_2/4190_Cyclophilin-A-Primary-antibodies-ab58144-5.jpg)