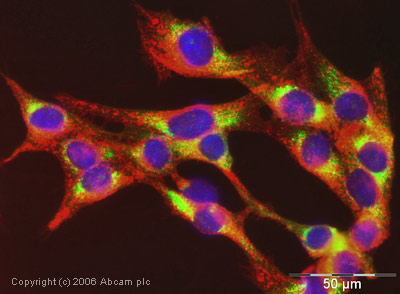
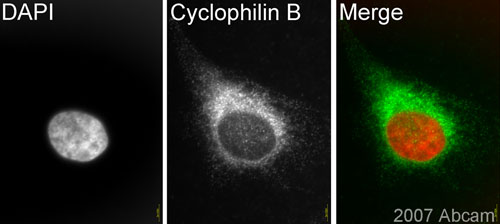
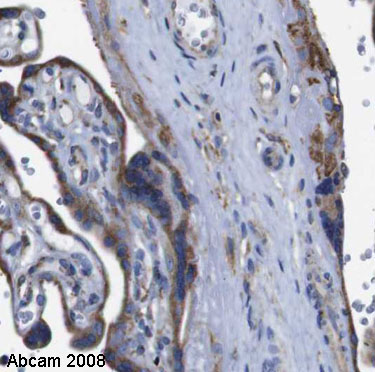
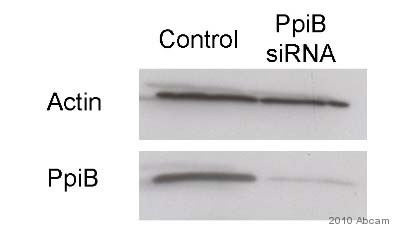
Anti-Cyclophilin B antibody
| Name | Anti-Cyclophilin B antibody |
|---|---|
| Supplier | Abcam |
| Catalog | ab16045 |
| Prices | $401.00 |
| Sizes | 100 µg |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Applications | WB IHC-P ICC/IF ICC/IF IHC-F IP |
| Species Reactivities | Mouse, Rat, Horse, Chicken, Dog, Human, Bovine, Pig, Xenopus |
| Antigen | Synthetic peptide conjugated to KLH derived from within residues 150 to the C-terminus of Human Cyclophilin B |
| Description | Rabbit Polyclonal |
| Gene | PPIB |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
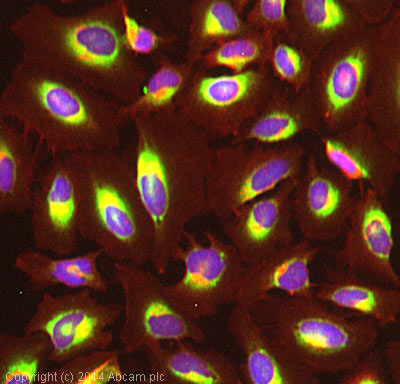
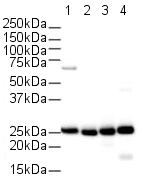
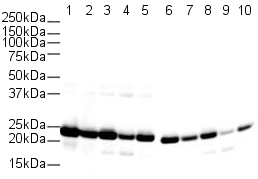
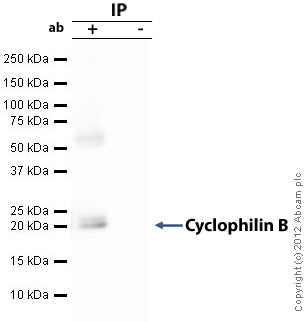
Product images
Product References
Tubular p53 regulates multiple genes to mediate AKI. - Tubular p53 regulates multiple genes to mediate AKI.
Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. J Am Soc Nephrol. 2014 Oct;25(10):2278-89.
Depletion of cyclophilins B and C leads to dysregulation of endoplasmic reticulum - Depletion of cyclophilins B and C leads to dysregulation of endoplasmic reticulum
Stocki P, Chapman DC, Beach LA, Williams DB. J Biol Chem. 2014 Aug 15;289(33):23086-96.
Cyclophilin A associates with enterovirus-71 virus capsid and plays an essential - Cyclophilin A associates with enterovirus-71 virus capsid and plays an essential
Qing J, Wang Y, Sun Y, Huang J, Yan W, Wang J, Su D, Ni C, Li J, Rao Z, Liu L, Lou Z. PLoS Pathog. 2014 Oct 2;10(10):e1004422.
Amyloid-beta oligomers induce differential gene expression in adult human brain - Amyloid-beta oligomers induce differential gene expression in adult human brain
Sebollela A, Freitas-Correa L, Oliveira FF, Paula-Lima AC, Saraiva LM, Martins SM, Mota LD, Torres C, Alves-Leon S, de Souza JM, Carraro DM, Brentani H, De Felice FG, Ferreira ST. J Biol Chem. 2012 Mar 2;287(10):7436-45.
Proteomic assessment shows that many endoplasmic reticulum (ER)-resident proteins - Proteomic assessment shows that many endoplasmic reticulum (ER)-resident proteins
Pehar M, Lehnus M, Karst A, Puglielli L. J Biol Chem. 2012 Jun 29;287(27):22436-40.
Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors - Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors
Wolff NC, Vega-Rubin-de-Celis S, Xie XJ, Castrillon DH, Kabbani W, Brugarolas J. Mol Cell Biol. 2011 May;31(9):1870-84.
Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and - Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and
Pyott SM, Schwarze U, Christiansen HE, Pepin MG, Leistritz DF, Dineen R, Harris C, Burton BK, Angle B, Kim K, Sussman MD, Weis M, Eyre DR, Russell DW, McCarthy KJ, Steiner RD, Byers PH. Hum Mol Genet. 2011 Apr 15;20(8):1595-609.
Novel embryonic neuronal migration and proliferation defects in Dcx mutant mice - Novel embryonic neuronal migration and proliferation defects in Dcx mutant mice
Pramparo T, Youn YH, Yingling J, Hirotsune S, Wynshaw-Boris A. J Neurosci. 2010 Feb 24;30(8):3002-12.
Stoichiometry of expressed alpha(4)beta(2)delta gamma-aminobutyric acid type A - Stoichiometry of expressed alpha(4)beta(2)delta gamma-aminobutyric acid type A
Wagoner KR, Czajkowski C. J Biol Chem. 2010 May 7;285(19):14187-94.
Analysis of low abundance membrane-associated proteins from rat pancreatic - Analysis of low abundance membrane-associated proteins from rat pancreatic
Borta H, Aroso M, Rinn C, Gomez-Lazaro M, Vitorino R, Zeuschner D, Grabenbauer M, Amado F, Schrader M. J Proteome Res. 2010 Oct 1;9(10):4927-39.