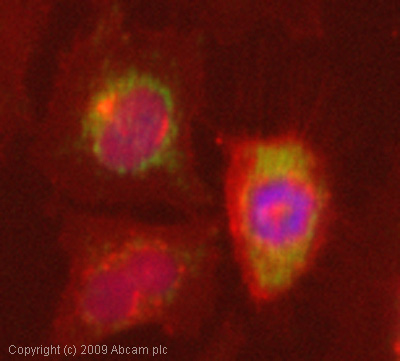
ICC/IF image of ab50050 stained HeLa cells. The cells were 4% formaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab50050, 1µg/ml) overnight at +4°C. The secondary antibody (green) was Alexa Fluor® 488 goat anti-mouse IgG (H+L) used at a 1/1000 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.
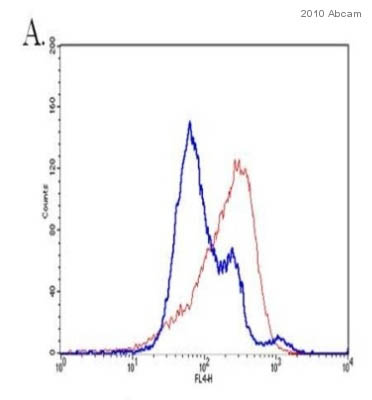
HeLa cells (3-4 x10E6/10cm dishmL) were either treated with DMSO (blue) or 100 mg/mL cycloheximide (red) for 16-18 hours. After permeabilization and fixation, cells were split into half in 1.5 mL tubes and stained for cytochrome C using ab50050. Secondary antibody was an AlexaFluor 633 anti-mouse secondary antibody. Cytochrome C can be seen to be released from the mitochondria by the shift in fluorescent intensity compared to DMSO control. Data was acquired on a FACSCaliber flow cytometer with CellQuest software.See Abreview

IHC image of Cytochrome C staining in human skeletal muscle formalin fixed paraffin embedded tissue section, performed on a Leica BondTM system using the standard protocol F. The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH6, epitope retrieval solution 1) for 20 mins. The section was then incubated with ab50050, 5µg/ml, for 15 mins at room temperature and detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The section was then counterstained with haematoxylin and mounted with DPX. For other IHC staining systems (automated and non-automated) customers should optimize variable parameters such as antigen retrieval conditions, primary antibody concentration and antibody incubation times.


