Anti-Cytochrome C antibody [7H8.2C12]
| Name | Anti-Cytochrome C antibody [7H8.2C12] |
|---|---|
| Supplier | Abcam |
| Catalog | ab13575 |
| Prices | $400.00 |
| Sizes | 100 µg |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG2b |
| Clone | 7H8.2C12 |
| Applications | FC IHC-F ICC/IF ICC/IF WB IHC-P |
| Species Reactivities | Mouse, Rat, Horse, Pigeon, Human, Drosophila |
| Antigen | Synthetic peptides corresponding to amino acids 1-80, 81-104 and 66-104 of pigeon CYT |
| Description | Mouse Monoclonal |
| Gene | CYCS |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Calcium concentration response to uterine ischemia: a comparison of uterine - Calcium concentration response to uterine ischemia: a comparison of uterine
Yang W, Cheng Z, Dai H. Eur J Obstet Gynecol Reprod Biol. 2014 Mar;174:123-7. doi:
The expression of apoptosis inducing factor (AIF) is associated with - The expression of apoptosis inducing factor (AIF) is associated with
Yu W, Bonnet M, Farso M, Ma K, Chabot JG, Martin E, Torriglia A, Guan Z, McLaurin J, Quirion R, Krantic S. BMC Neurosci. 2014 Jun 10;15:73.
Phosphorylation-dependent mitochondrial translocation of MAP4 is an early step in - Phosphorylation-dependent mitochondrial translocation of MAP4 is an early step in
Hu J, Chu Z, Han J, Zhang Q, Zhang D, Dang Y, Ren J, Chan HC, Zhang J, Huang Y. Cell Death Dis. 2014 Sep 18;5:e1424.
Meckelin 3 is necessary for photoreceptor outer segment development in rat Meckel - Meckelin 3 is necessary for photoreceptor outer segment development in rat Meckel
Tiwari S, Hudson S, Gattone VH 2nd, Miller C, Chernoff EA, Belecky-Adams TL. PLoS One. 2013;8(3):e59306.
Identification of mitochondrial dysfunction in Hutchinson-Gilford progeria - Identification of mitochondrial dysfunction in Hutchinson-Gilford progeria
Rivera-Torres J, Acin-Perez R, Cabezas-Sanchez P, Osorio FG, Gonzalez-Gomez C, Megias D, Camara C, Lopez-Otin C, Enriquez JA, Luque-Garcia JL, Andres V. J Proteomics. 2013 Oct 8;91:466-77.
Localization of HPV-18 E2 at mitochondrial membranes induces ROS release and - Localization of HPV-18 E2 at mitochondrial membranes induces ROS release and
Lai D, Tan CL, Gunaratne J, Quek LS, Nei W, Thierry F, Bellanger S. PLoS One. 2013 Sep 24;8(9):e75625.
Imaging of neurosphere oxygenation with phosphorescent probes. - Imaging of neurosphere oxygenation with phosphorescent probes.
Dmitriev RI, Zhdanov AV, Nolan YM, Papkovsky DB. Biomaterials. 2013 Dec;34(37):9307-17.
Inhibition of mitochondrial fission prevents cell cycle progression in lung - Inhibition of mitochondrial fission prevents cell cycle progression in lung
Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C, Archer SL. FASEB J. 2012 May;26(5):2175-86.
Bid signal pathway components are identified in the temporal cortex with - Bid signal pathway components are identified in the temporal cortex with
Jiang H, He P, Adler CH, Shill H, Beach TG, Li R, Shen Y. Neurology. 2012 Oct 23;79(17):1767-73.
Nitric oxide and calcium participate in the fine regulation of mitochondrial - Nitric oxide and calcium participate in the fine regulation of mitochondrial
Le Pennec S, Mirebeau-Prunier D, Boutet-Bouzamondo N, Jacques C, Guillotin D, Lauret E, Houlgatte R, Malthiery Y, Savagner F. J Biol Chem. 2011 May 20;286(20):18229-39.
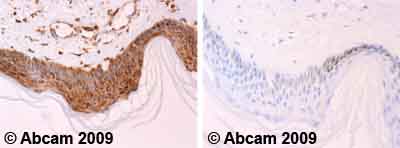
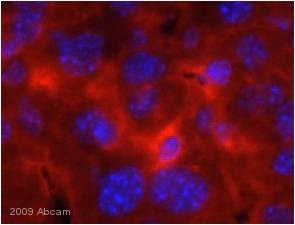
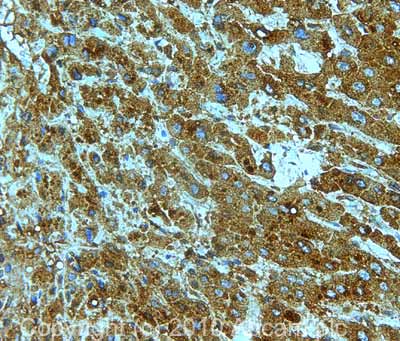
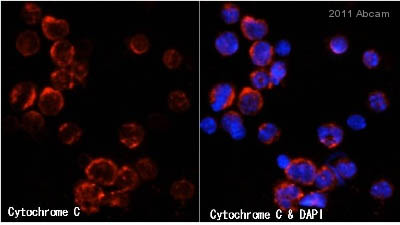
![All lanes : Anti-Cytochrome C antibody [7H8.2C12] (ab13575) at 1 µg/mlLane 1 : HeLa (Human epithelial carcinoma cell line) Whole Cell Lysate Lane 2 : Jurkat (Human T cell lymphoblast-like cell line) Whole Cell Lysate Lane 3 : Heart (Human) Tissue Lysate - adult normal tissue (ab29431)Lysates/proteins at 10 µg per lane.SecondaryGoat Anti-Mouse IgG H&L (HRP) preadsorbed (ab97040) at 1/5000 dilutiondeveloped using the ECL techniquePerformed under reducing conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_2/4704_Cytochrome-C-Primary-antibodies-ab13575-37.jpg)
![Overlay histogram showing HepG2 cells stained with ab13575 (red line). The cells were fixed with 80% methanol (5 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab13575, 0.1µg/1x106 cells) for 30 min at 22ºC. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG1 [ICIGG1]/mouse IgG2b [PLPV219] (ab91353/ab91366, 1µg/1x106 cells) used under the same conditions. Unlabelled sample (blue line) was also used as a control. Acquisition of >5,000 events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_2/4705_ab13575-20881-ab13575AP1586532FC.jpg)