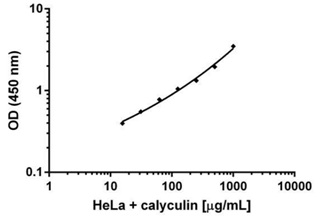
The extract was prepared with HeLa cells treated with 50nM of calyculin for 15 minutes. Data was plotted on an XY graph in the log scale and a four parameter algorithm formula was used for curve fitting.
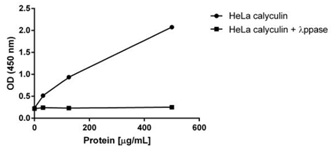
Calyculin treated HeLa extracts were left untreated (control) or treated with 1:100 dilution of ? Ppase at 34°C. Samples were loaded at 500, 125 and 30 µg/mL on the plate and measured following the kit’s protocol. Treatment of calyculin treated HeLa extracts with ? Ppase decreases the signal by 12 fold at the highest loading.
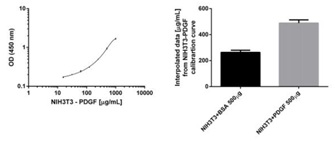
NIH3T3 cells were treated with 50 ng/mL of PDGF recombinant protein after serum starvation or BSA control for 30 minutes at 37°C. A protein titration of PDGF treated cells was loaded in triplicate (left panel) alongside 4 replicates of both BSA and PDGF treated cells at 500 µg/mL. BSA and PDGF samples were interpolated from the standard curve and results are shown on the right panel. PDGF increases the levels of phosphorylation at Ser2448 by 2 fold.
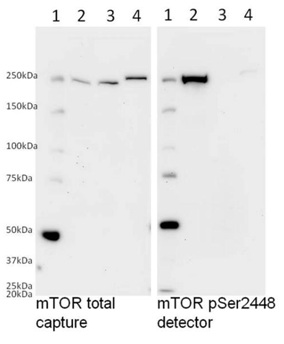
The detector antibody used in this kit specifically detects the phosphorylated mTOR as determined by Western blotting. Samples were loaded on a 8% polyacrylamide gel as follows: (1) Marker (2) calyculin treated HeLa lysate, (3) calyculin treated HeLa lysate dephosphorylated with 1:100 dilution of ? Ppase at 34°C, (4) Hek293T cell lysate. Samples were then diluted in SDS-PAGE buffer and loaded at 40 µg/well. Membranes were blocked with 20% blocking buffer (ab126587) in TBST for 1 hour and incubated with either the detector antibody mTOR pSer2448 or the capture antibody against total mTOR in 10% blocking buffer (ab126587) in TBST overnight. Labeling was carried out with secondary antibodies conjugated to HRP. Treatment with calyculin in HeLa cells induces phosphorylation at Ser2448 and ? Ppase completely dephosphorylates mTOR.



