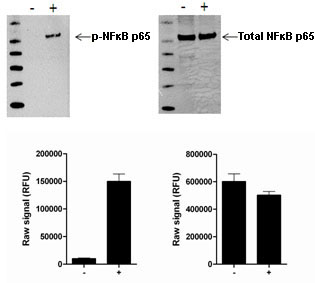
Using the NFKappaB p65 assay kits, a significant stimulation of NFKappaB p65 phosphorylation at Ser536 is detected in HeLa cells treated with TNF alpha for 10 minutes compared with untreated cells, while no change in total NFKappaB p65 levels is observed.
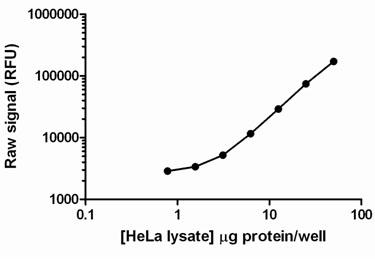
Using the PhosphoTracer phospho-NFKappaB p65 assay, the amount of lysate required to detect NFKappaB p65 in TNF alpha-treated HeLa cell lysates was examined. Up to 50 µg of lysate/well was loaded into replicate wells of a PhosphoTracer microplate, and analysed for phospho-NFKappaB p65. Phospho-NFKappaB p65 was readily detected in less than 10 µg of lysate/well.
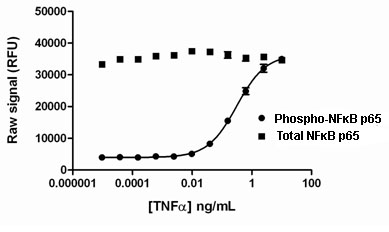
Day 1: HeLa cells were seeded at 25K cells/well in a 96 well tissue culture plate in medium containing 10% FBS, and incubated overnight. Day 2: The media was removed and cells were stimulated with various concentrations of TNF aplha for 10 minutes. The media was removed, and the cells were lysed with 125 µL/well freshly prepared Lysis Mix, with shaking for 10 min. Lysates (50 µL) were transferred to replicate wells of an PhosphoTracer assay plate and analyzed for either phospho or total NFKappaB p65 using the standard PhosphoTracer protocol. Briefly, Antibody Mix specific for either phospho-NFKappaB p65, or Total NFKappaB p65 (50 µL/well), was added to the lysate, and the plate was incubated for 1 hr at room temp, with shaking. The plate was washed and Substrate Mix was added to the wells. The plate was covered in foil and incubated for 10 min with shaking. Signal in the wells was determined using a plate reader.


