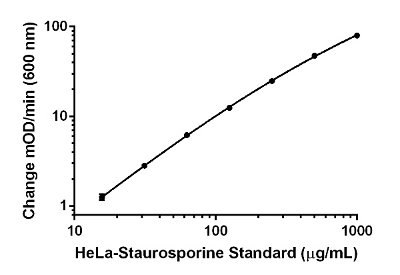
HeLa-Staurosporine standard curve. The HeLa Staurosporine standard was prepared using HeLa cells treated for 4 hours with 1 µM staurosporine (ab120056). Background-subtracted data values (mean +/- SD) are graphed.
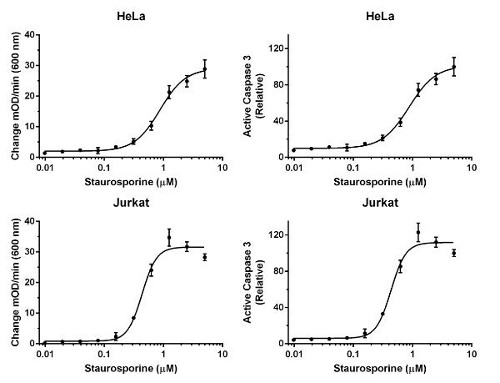
Lysates corresponding to 0.33x106 HeLa cells/mL or 0.50x106 Jurkat cells/mL were prepared by direct in-well lysis (without media removal) from cells treated for 4 hours with variable doses of staurosporine in a 96-well plate. 100 µL of the lysates were analyzed in triplicates. Background-subtracted signals are shown in left panels. Relative active caspase 3 concentrations interpolated from standard curves and expressed as percent of cells treated with the highest dose of staurosporine are shown in right panels. IC50 were 0.9 µM for HeLa cells and 0.4 µM for Jurkat cells.
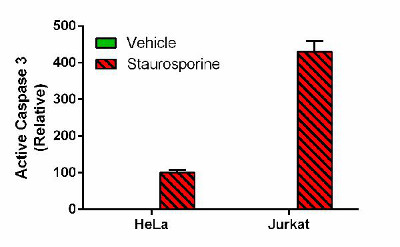
Demonstration of the assay specificity by induction of active caspase 3 by staurosporine treatment. Cells were treated for 4 hours with 1 µM staurosporine or drug’s vehicle (DMSO) and 500 µg/mL of HeLa cell extracts and 125 µg/mL Jurkat cell extracts prepared from cell pellets were analyzed using this kit. Relative active caspase 3 levels were interpolated from HeLa-Staurosporine standard curve and expressed as percent of staurosporine-treated HeLa cell. Note that no active caspase 3 (p17 subunit) was detected in vehicle-treated HeLa and Jurkat cells.
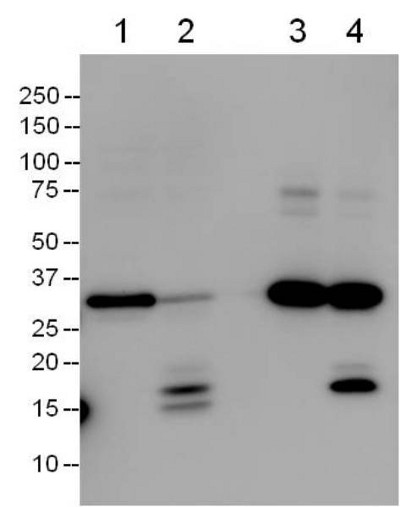
Cell extracts were prepared from 4 hours vehicle-treated (lanes 1 and 3) and 1 µM staurosporine-treated (lanes 2 and 4) Jurkat cells. Extracts were incubated with the Caspase 3 Microplate, captured proteins were extracted and analyzed by Western blotting using a pro+p17 caspase 3 antibody ab32351 (Lanes 3 and 4). 25% of the amounts of the extracts used for immunocapture were also analyzed directly by the Western blotting (lanes 1 and 2). Note that the Caspase 3 Microplate captures both the pro- and p17 subunit of caspase 3.



