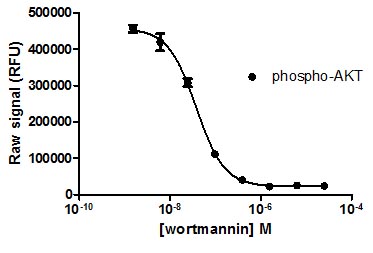
Jurkat cells were harvested by centrifugation, resuspended in HBSS, and seeded at 10K/well (20 µL/well) in a rinsed PhosphoTracer assay plate. The cells were treated with various concentrations of wortmannin (10 µL/well) for 1 h at 37°C, then treated with 5% FCS (10 µL/well) for 15 min. The cells were lysed with 10 µL/well Lysis Mix Concentrate, with shaking for 10 min. Antibody Mix specific for phospho AKT (50 µL/well) was added to the lysate, and the plates were incubated for 1 hr at room temp, with shaking. Plates were washed and Substrate Mix was added. The plates were covered in foil and incubated for 10 min with shaking. Signal in the wells was determined using a plate reader.
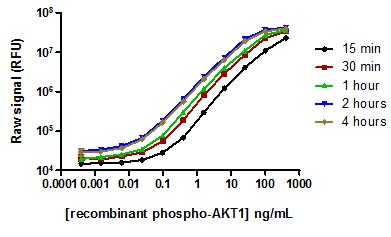
Using the PhosphoTracer phospho-AKT assay, the length of assay incubation time that was required to detect recombinant phospho-AKT was examined. After 15 mins, phospho-AKT concentrations of approximately 100 pg/mL were readily detected, while after 1 hour incubation, the limit of detection was less than 10 pg/mL. Longer incubation times had little effect on overall limit of detection that was observed.
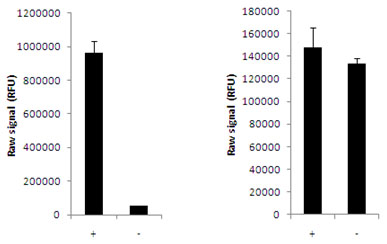
Using the AKT 1/2/3 assay kits, a significant increase in AKT 1/2/3 phosphorylation at Ser473 is detected in MCF7 cells treated with insulin for 15 minutes (+), compared with untreated cells (-), whereas no change in total AKT 1/2/3 levels was observed.
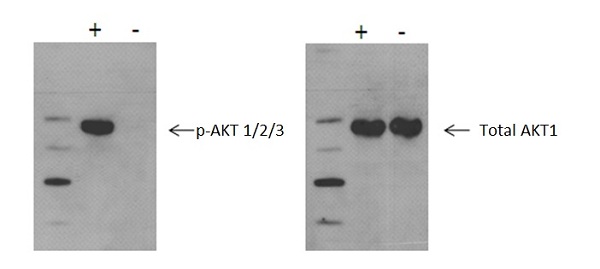
Using Western blot, a significant increase in AKT 1/2/3 phosphorylation at Ser473 is detected in MCF7 cells treated with insulin for 15 minutes (+), compared with untreated cells (-), whereas no change in total AKT 1/2/3 levels was observed.



