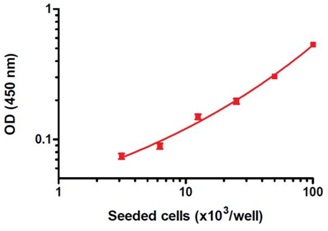
Dynamic range of the ab139410 assay kit. HeLa cells were allowed attach overnight and treated for 4 hours with 1 µM staurosporine (ab120056). The data, presented as background-subtracted OD 450 nm values (mean +/-SEM), were obtained using this kit as described in the protocol.
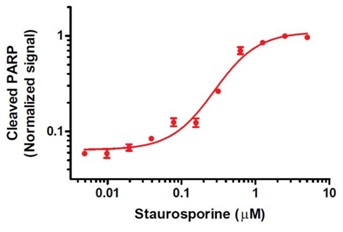
Example of determination of staurosporine EC50 of the PARP cleavage using ab139410 assay kit. HeLa cells, seeded at 50,000 per well were allowed attach overnight and treated for 6 hours with staurosporine (ab120056) as indicated. The data, presented as Janus Green normalized background-subtracted OD 450 nm values (mean +/-SEM), were obtained using this kit as described. The determined staurosporine EC50 value is 0.28 µM.
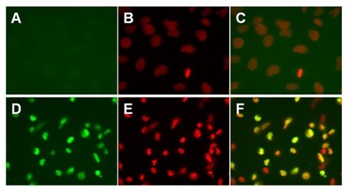
Cleaved PARP antibody specificity demonstrated by immunocytochemistry. HeLa cells were treated with vehicle (A, B and C) or 1 µM Staurosporine (ab120056, D, E, and F) for 4 hours to induce apoptosis. The cells were treated, permeabilized, blocked and incubated with the Cleaved PARP antibody as described in the protocol of this kit. Samples were further processed for fluorescence immunocytochemistry and co-stained with DNA stain DAPI. Images of green Cleaved PARP signals (A and D), red DAPI signals (B and E) and overlay of these green and red images (C and F) are shown. Note that the Cleaved PARP antibody specifically labels nuclei of staurosporine-treated cells.
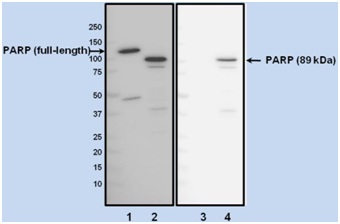
Figure 4. Cleaved PARP antibody specificity demonstrated by Western blotting. Western blot analysis of HeLa cells treated with vehicle (DMSO, lane 1 and 3) or 1 µM staurosporine (lanes 2 and 4) for 4 hours to induce apoptosis. Blots were incubated with an antibody that recognizes both the full-length PARP-1 and its 89 kDa fragment (left panel), or with the Cleaved PARP antibody used in this kit (right panel). Appropriate HRP-conjugated secondary antibodies followed by ECL detection were used. Note that the Cleaved PARP antibody recognizes the apoptosis-specific 89 kDa fragment of PARP but it does not recognize the 113 kDa full-length PARP.
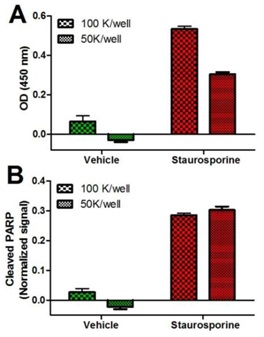
Specificity of the ab139410 assay kit. HeLa cells, seeded at indicated densities, were allowed attach overnight and treated for 4 hours with drug vehicle (DMSO) or 1 µM staurosporine (ab120056) to induce apoptosis. The data (mean +/-SEM), presented as background-subtracted OD 450 nm values (A), or Janus green-normalized signals (B), were obtained using this kit as described in the protocol.




